Biology Reference
In-Depth Information
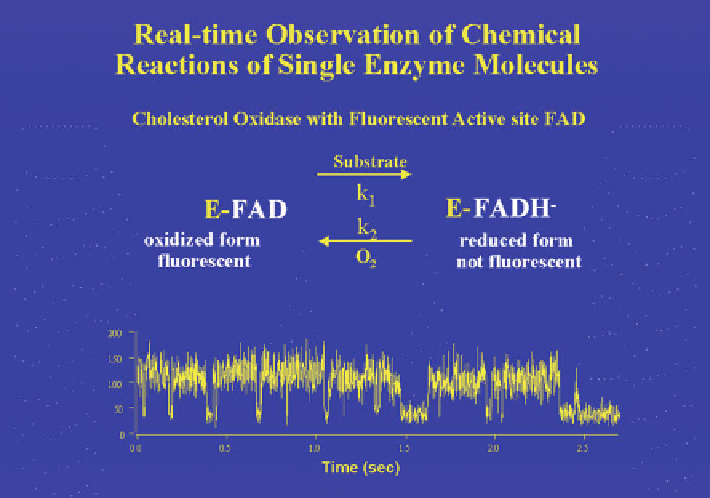
Fig. 11.17 The measurement of the turnover of a cholesterol oxidase (COx) molecule in the
presence of cholesterol (0.20 mM) and oxygen (0.25 mM). The prosthetic group, FAD, is
fluorescent when in its oxidized state with an average relative intensity of about 130 units
(which is referred to as the “on” state) and nonfluorescent when in its reduced state with an
average intensity of about 40 units (which is referred to as the “off” state) (Reproduced from
http://
The relative importance of these pathways during a given cycle of photon
absorption and emission probably depends on the conformation of FAD molecule
which is in turn most likely affected by the local conformational structure of
the FAD binding pocket of COx. It is for this reason that, as the conformation of
COx fluctuates, the fluorescence efficiency, defined as k
g
/( k
g
+
k
nr
+
k
ET
)
, also
fluctuates, thus accounting for the fluorescence fluctuations observed during the
on- or off-times as shown in Fig.
11.17
, or between A and B and between C and D
in Fig.
11.20
.
Lu et al. (1998) used a single-molecule manipulation technique (Xie 2001; Ishii
and Yanagida 2007) to measure the cycling of cholesterol oxidase between its
oxidized (“on”) and reduced (“off”) states (Fig.
11.17
). Two of the most significant
findings Lu et al. (1998) made are (a) that the enzyme molecule spends variable
times in on- or off-states and (b) that the on- and off-times are not distributed
randomly (or normally) but have a long tail. As can be seen on the lower left corner
of Fig.
11.18
, the on-times varied from about 70 ms (milliseconds) to 1,300 ms with
most probable on-times lying between 100 and 200 ms. Estimating from the area
under the “on-time” distribution histogram in Fig.
11.18
, it may be concluded that