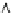Biology Reference
In-Depth Information
Fig. 8.7
The molecular mechanism of the action of the ATP-driven calcium ion pump of
sarcoplasmic reticulum represented as a
bionetwork
consisting of four nodes and four edges.
The nodes of the network represents the structural and chemical states of the
C
and
T
domains of
the pump (that are mechanically coupled as indicated by ~) and the edges represent the state
transitions and associated movements of ligands in and out of their binding sites
3. In State
III
, the
C
domain is phosphorylated and the Ca
++
-binding site becomes
inaccessible from either the cytoplasmic or luminal side and the calcium-binding
affinity of the
T
domain decreases.
4. In State
IV
, the
C
domain releases ADP leaving the phosphoryl group covalently
bound to
C
while the
T
domain opens toward the luminal side, releasing Ca
++
by
lowering its Ca
++
-binding affinity.
There are two basic factors operating in Fig.
8.7
that control the activity of the
calcium ion pump (and all other molecular machines for that matter). One is the
thermodynamic
factor that determines the direction of the net ion movement across
the membrane, from a high free energy to the low free energy states, leading to a net
free energy decrease, and the other is the
kinetic
factors controlling the activation free
energy barriers that ions must overcome in order to move through the membrane and
hence the rates of transmembrane ion movement. Either factors alone are insufficient
to drive the ion movement; both conditions must be satisfied for ion movement (or
the motion of any goal-directed or purposive molecular machines). We may refer to
the first as the “thermodynamic requirement” and the second as the “kinetic require-
ment.” It is postulated here that the
thermodynamic
requirement is met by the Gibbs
free energy associated with the concentration gradients of ATP or Ca
++
ion and the
kinetic
requirement is satisfied by the
conformon-driven structural changes
of the
Ca
++
ATPase that modulate the local activation energy barriers for catalysis in
C
domain and ion transport through the
T
domain. This view can be stated as follows:
The direction of ion movement is determined by global thermodynamics of the exergonic
chemical reactions or physical processes, and the rate of ion movement is determined by
conformons generated in enzymes locally through ligand-binding processes. (8.19)
Statement 8.19 is consistent with the view that the primary role of enzymes and
molecular machines is to control
timing
or to effect
temporal structures
(see Sect.
Hypothesis of Active Transport.” It is possible to generalize Statement 8.19 so that