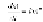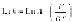Environmental Engineering Reference
In-Depth Information
less oil per unit mass, and PVC degradation produces corrosive HCl that has
to be removed during the process. Though it has never been demonstrated,
bio-based plastics such as poly(lactic acid) (PLA) should also be amenable
to conventional pyrolysis.
A carbonaceous char residue waste (2-13%), often contaminated with
catalyst residue and inorganic fillers, is formed in pyrolysis. This may be
either used as solid fuel, activated C, or disposed of in a landfill.
Mechanism of pyrolytic degradation is complex involving a large set of
reactions, even for a single class of plastic. Generic degradation reactions
such as chain scission, H-transfer, unzipping, disproportionation, and
combination occur in the process. Typical mix of products from mixed
plastic waste streams with different pyrolysis conditions are shown in
Table
Table 9.3
Yield of Products from Pyrolysis of Mixed Plastic
Min)
Source: Based on data from Lopez-Urionabarrenechea et al. (2012).
Pyrolysis method Liquid Gas Residue
Uncatalyzed pyrolysis 79.3 ± 1.9 17.7 ± 1.9 3.0 ± 0.3
Catalyzed pyrolysis (zeolite ZSM-5) 56.9 ± 3.0 40.4 ± 3.0 3.2 ± 0.2
Uncatalyzed followed by catalyzed 69.0 ± 0.1 29.0 ± 0.1 2.00 ± 0.0
Dehydrochlorination + catalytic
56.8 ± 2.0 41.2 ± 2.0 2.00 ± 0.2
a
PE = 40%, PP = 35%, PS = 18%, PET = 4, and PVC = 3.
The rate of loss in weight of the plastic can be described by first-order
kinetics (Marcilla et al., 2003), allowing an activation energy for the process
to be estimated and is as follows:
where,
m
is the mass of solid plastic left at time
t
,
n
is the order of reaction,
A
is the preexponential factor, and
E
is the activation energy. For PE, for
instance,
E
~ 250 kJ/mol and the reaction rate is very significantly affected