Environmental Engineering Reference
In-Depth Information
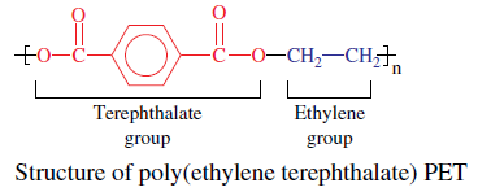
enough for use as bottle resins. Antimony trioxide is used as a catalyst in
polymerization (Duh, 2002).
Neither BPA nor phthalate is used in manufacturing, compounding, or
processing of PET into bottles. These cannot be generated as a by-product
during manufacture or by the degradation of PET during use. Therefore,
PET is regarded a relatively “safe” choice for packaging beverages.
Consistent with the expectation, clean PET bottles generally do not show
any EDCs or cytotoxic or genotoxic compounds leaching into water
contained in them (Bach et al., 2013; Pinto and Reali, 2009). Leaching of
trace levels of antimony catalysts into water has been reported (Keresztes,
2009).
Recent reports, however, claim PET-bottled water and beverages to show,
albeit very mild, but definitive ED activity (Sax, 2010). The effect, however,
is inconsistent (Ceretti et al., 2010; Guart et al., 2011) and not seen in all
bottles tested in a given study (Wagner and Oehlmann, 2009, 2011) and
in bottles purchased at retail stores (Pinto and Reali, 2009). The estimated
exposure to EDCs via this route, however, is very low (a pg to a ng estradiol
per day (Kereszteset al., 2009)).
In a 2009 study, mud snails (
Potamopyrgus antipodarum
) growing in
washed-out PET bottles for 56 days showed significant (
p
< 0.0001)
increase in embryo production (sign of ED activity equivalent to
approximately 25-75 ng/l of ethinyl estradiol) compared to those grown
on glass (Wagner and Oehlmann, 2009). Other studies (Montuori et al.,
2008; Pinto and Reali, 2009) show similar activity in yeast bioassay but at
a lower level (Farhoodi et al., 2008). Some studies suggest the phthalates or
other EDCs to have leached out of the PET (Bošnir et al., 2007; Casajuana
and Lacorte, 2003). But phthalate contamination of some bottled water has
been reported (Criado et al., 2005; Farhoodi et al., 2008; Montuori et al.,