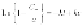Environmental Engineering Reference
In-Depth Information
(6.6)
For bleached paperboard for instance, Andrady et al. found
k
~ (0.14-0.18)
days
−1
(Andrady and Song, 1999).
In practice, 100% of a plastic material will not mineralize and a small
residue remains in the environment. These may include fillers, catalyst
residues, and recalcitrant additives. It is important to ensure that these and
their reaction products are non-toxic and do not harm soil organisms or
affect plant growth.
6.5 BIODEGRADABILITY OF COMMON POLYMERS
Slow biodegradation of common polymers such as PE, PET, or PP does
occur in nature (Shah et al., 2008; Tsao et al., 1993) but at rates that are
several orders of magnitude lower than that observed with biopolymers
such as cellulose and chitosan or biodegradable synthetic polymers such
as PCL. These rates are too low to have any significant impact on litter
management, and conventional plastics as are not usually thought of or
referred to as being “biodegradable.” The few microorganisms capable of
biodegrading these are usually not abundant in natural settings and are
often overwhelmed by native species.
Plastics in soil or water environments develop a microbe-rich biofilm on
the surface. Polyethylene, the plastic used in highest volume, when exposed
to seawater, soil, or other biotic environment is readily populated on the
surface by a consortium of microorganisms (see
Fig. 6.16
; Zettler et al.,
2013). Gilan et al. (2004) attributed the biofilm formation to slow reduction
in hydrophobicity of the surface. Microscopic examination of the surface
of plastics exposed to biotic environments (for instance marine sediment)
for longer periods of time show surface pits and depressions where
microorganisms appear to have settled in and biodegraded the surface
(Bonhomme et al., 2003). The pitted and eroded area under the foot of an
attached organism is believed to be biodegraded (Bonhomme et al., 2003),
and the local molecular weight was shown to be lower than that of the
surrounding polymer (Ohtaki et al., 1998). The rate of this degradation
is very slow because the species of interest are not major constituents in
natural consortia and also because of the availability of alternative carbon
sources that are easier to digest and assimilate by microorganisms.