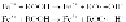Environmental Engineering Reference
In-Depth Information
Accelerated photodegradation is achieved either using structural
modifications of the resin itself or by using catalysts or additives that
accelerate the oxidation reactions (Koutny et al., 2006; Wiles and Scott,
2006). A copolymer of ethylene with approximately 1% of carbon monoxide
is an example of a photodegradable plastic (Scoponi et al., 1993). Carbonyls
are potent chromophores that absorb solar UV-B radiation (Andrady et
al., 1993a). On absorbing the radiant energy, the copolymer undergoes
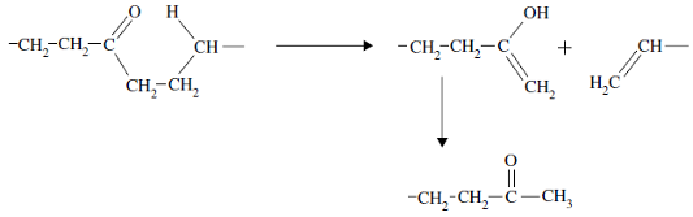
photolysis via the Norrish II reaction leading to chain scission and therefore
group is needed in the structure to effect this reaction as shown below.
Norrish II reaction was also suggested in recent work on photodegradation
of PLA (Tsuji et al., 2006).
Asecondapproachusestransitionmetalpro-oxidantcatalystsasanadditive
(Pablos et al., 2010; Roy et al., 2009). Several “photodegradable” and
“oxo-biodegradable” plastic products rely on this approach. Transition
metals can act as redox catalysts to accelerate degradation via catalyzed
peroxide decomposition into radicals.
Typically,transitionmetals,suchasmanganese,iron,cobalt,andnickel(but
not heavy metals), are used to catalyze peroxide decomposition (Andrady et
al., 1996). Their additive masterbatches are used at very low levels, and the
metals are therefore present at very low concentrations of about 0.01 and
0.5%weightintheplasticproduct(Arnaudetal.,1994).Someoftheplastics
with these additives have been approved for use in food contact plastics in
the United States and in Canada.