Environmental Engineering Reference
In-Depth Information
1. Absorbing incident UV radiation using organic (e.g., with
benzophenones and benzotriazoles) or inorganic (e.g., rutile titanium
dioxide) additives.
2. Quenching the photo-excited species formed in the polymer (e.g., with
nickel dibutyl dithiocarbamate).
3. Removing the free radicals formed in the polymer (e.g., with hindered
amine light stabilizers (HALS)).
The first two classes of compounds inhibit initiation reactions that form
radical products, while the third mops up the radicals after they are formed.
With rigid PVC products used in exterior building applications,
approximately 9-13% of rutile titanium dioxide is often used as an opacifier
pigment. The pigment absorbs incident UV radiation shielding the bulk
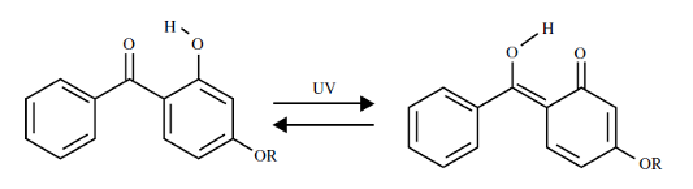
polymer from exposure. Organic UV absorbers such as benzophenone or
benzotriazoles compete with the chromophores in the plastic in absorbing
radiation. For instance, benzophenones reversibly isomerize in the process.
HALS are by far the most efficient class of stabilizer for polyolefins and
are not consumed in the stabilization process (Gugumus, 1991; Malik et
al., 1995). They work via the nitroxyl radical species that reacts with RO·
shown in
Figure 6.7
, making it a very effective radical-mop even at very
low concentrations (Gugumus, 2002a). These can be used only at levels of
0.05% by weight and still deliver excellent protection against UV damage
in polyolefins. HALS cannot be used in PVC formulations as HCl from
dehydrochlorination deactivates them.