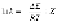Environmental Engineering Reference
In-Depth Information
material. Litter is believed to fragment into progressively smaller pieces by
this mechanism until the size range is small enough for it to blend in with
the sand or soil in the background. Plastic fragments mixed in with the soil
or water can then undergo slow biodegradation.
Fragmentation is an important precursor to biodegradation as it increases
the surface area of plastic available to microbes to interact with. However,
the kinetics of fragmentation (as opposed to that of degradation) and the
evolution of particle sizes during fragmentation of plastics outdoors are
essentially unknown.
Adistinctionneedstobemadebetweendegradation-induced fragmentation
described earlier and that due to entirely physical causes. Marine borers
consuming polystyrene (EPS) foam floats (Davidson, 2012) and insects or
rodents attacking plastic cable covers and gas pipes also create small plastic
fragments (Chalot, 2011). But neither the average molecular weight
Mn
(g/
mol) or the chemical structure of the polymer are altered in these processes.
Therefore, not being true degradation, these changes are referred to as
“biodeterioration.” The same is true of composite plastics mixed with
(Sunilkumar et al., 2012; Rutkowska et al., 2002) blends that are sometimes
mislabeled as “biodegradable plastics.” On environmental exposure, only
the starch or chitosan fraction in these will readily biodegrade, and the
resulting porous recalcitrant LDPE residue crumbles into small fragments
(Jbilou et al., 2013). The
Mn
(g/mol) of the LDPE particles so produced is
not reduced in the process.
6.2.3 Temperature and Humidity Effects on Degradation
In common with all chemical reactions, the rate of oxidative degradation
also increases with the temperature of the substrate (Andrady et al., 2003).
The magnitude of acceleration depends on the activation energy of the
degradation process. The common form of the Arrhenius equation relates
the rate constant
k
of a reaction to the temperature
T
(K).
(6.1)
where
R
is the gas constant (1.986 cal/mol),
E
is the activation energy, and
Z
is a constant. Therefore,