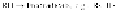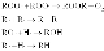Environmental Engineering Reference
In-Depth Information
scheme involved is very well established and is schematically shown in
1. Initiation:
2. Propagation:
3. Termination:
As only hydrogen atoms in the polymer are abstracted in the reaction
sequence, the polymer is designated as RH. The initiation reaction yields
R· or alkyl radicals that add to molecular oxygen to form peroxy radicals
(ROO·) that in turn reacts with the polymer (RH) to form hydroperoxide
(ROOH) and an R· radical. As long as there is a steady supply of RH and
oxygen, these two reactions can go on to convert the available -RH to
-ROOH functionalities. The -ROOH moieties themselves undergo
photolysis to yield RO· radicals making this an autocatalytic process. Some
radicals may combine pairwise at high enough concentrations, or get
otherwisedeactivated,toendthecyclicprocess.Athighextentsofoxidation,
the initial reaction products themselves get oxidized leading to the
formation of a mix of aldehydes, carboxylic acids, ketones, and alcohols
(Andradyetal.,2007).Thismakesthecarbonylindex(theratioofthe>C=O
band at 1740 cm
−1
to the “thickness” band at 2020 cm
−1
in infrared (IR)
spectrum of the polymer) that increases with weathering (Andrady et al.,
1993b; Roy et al., 2007), a convenient spectroscopic means of quantifying
oxidation.
Chain scission accompanying these oxidative reactions, believed to occur
during the propagation step of the sequence, is mostly responsible for the
loss of useful properties in the polymer. As these reactions generate a high
concentration of polymer radicals, some cross-linking (joining of chain
radicals) can also occur concurrently with chain scission. But chain scission
usually dominates over cross-linking in polyolefins exposed to
solar-simulated radiation (Craig et al., 2005). Photo-oxidized polyolefins