Environmental Engineering Reference
In-Depth Information
mol) or sometimes with a higher average cross-link density, but always with
markedly compromised useful properties, is obtained.
6.2 CHEMISTRY OF LIGHT-INDUCED DEGRADATION
Plastics used outdoors are routinely exposed to sunlight and undergo facile
light-induced degradation (Hamid et al., 1995; Ranby, 1989; Sheldrick and
Vogl, 2004). This is often the only significant degradation mechanism
relevant to urban plastic litter and plastics used outdoors, such as in the
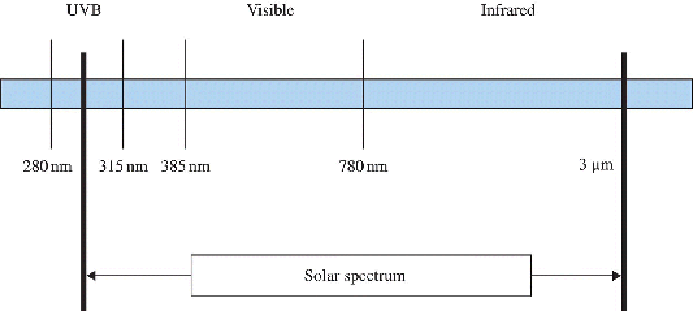
exterior of buildings. Solar radiation at the Earth's surface, facilitating
degradation, can be divided into three broad regions as shown in
Figure 6.2
.
It is only the visible-light wavelengths in the spectrum that can be perceived
by the human eye but it is the solar UV-B radiation (
λ
= 290-315 nm)
that is the most efficient in initiating degradation reactions (Torikai, 2000).
Terrestrial solar UV spectrum contains only a small (~5%) fraction of UV-B
radiation, most of it being filtered out by the stratospheric ozone layer. The
spectralirradiancedistributionofsunlightaswellasthetotalsolarradiation
dose varies widely with the geographic location. In places such as Arizona,
it can be as high as 8,000 MJ with approximately 330 MJ (total UVR) per
year.
Figure 6.2
Regions of the solar spectrum reaching the Earth's surface.
An important prerequisite for light-induced degradation is that the plastic
degrade the plastic. Polyolefins in theory do not have chromophores that