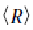Environmental Engineering Reference
In-Depth Information
Young RJ, Lovell PA.
Introduction to Polymers
. Boca Raton: CRC Press;
2011.
Notes
1
Thegroup“polymers”includeplastics,rubbers,andfibers.But,wewilluse
the terms polymer and plastic interchangeably in this work.
2
With poly(vinyl chloride) or poly(methyl methacrylate), however, the
parentheses should always be used because the term without them is
ambiguous. Writing all polymer names with parenthesis (e.g.,
poly(ethylene)) would still be correct.
3
However, the contour length
L
of the chain is not as useful a measure of
the “size” of polymer molecules as . An alternate measure of the size of
apolymermoleculeistherootmeansquareofits“radiusofgyration,”Rg.
It is defined as the average distance of a chain element from the center of
gravity of the chain.
4
The mean end-to-end distance
for such a chain is zero. Therefore, the
second moment is used instead.
5
A mole (abbreviated mol.) is a number like a dozen or a gross. It is a large
number: 6.022 × 10
23
. The molecular weight of any compound is the
mass in grams of the above large number of molecules of that compound.
This number is called Avogadro's number.
6
In a polymer chain where all backbone carbon atoms have a tetrahedral
molecular geometry, the zigzag backbone is in the plane of the paper with
the substituents either sticking out of the paper or retreating into the
paper.
7
Melting point of semicrystalline plastics should not be confused with the
term “melt” as used in plastic processing. The latter is the viscous liquid
obtained when plastics are heated to high processing temperatures. A
great majority of plastics, even amorphous ones, heated to high enough
temperatures will form viscous “melts.”