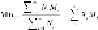Environmental Engineering Reference
In-Depth Information
as a collection, these long, flexible molecules are highly entangled and
strongly attracted to each other to form a unique solid.
3.2 CONSEQUENCES OF LONG-CHAIN MOLECULAR
ARCHITECTURE
This long-chain molecular geometry has interesting consequences in terms
of properties and is invariably the reason behind the success of plastics as
a material. Some important practical consequences of linear architecture of
molecules will be considered in this section.
3.2.1 Molecular Weight of Chain Molecules
When a collection of monomer molecules such as ethylene is converted
into polymer, the result is a mix of long-chain molecules. Different
macromolecules in the mix will have a different chain length; their
individual values of
n
will be different. But since each PE molecule can be
representedbythesamechemicalstructure,-(CH
2
-CH
2
-)
n
,theyareallPE
molecules but with different molecular weights. Unlike organic compounds
such as ethylene (mol. wt. = 28), table salt (mol. wt. = 58.54), or ammonia
(mol. wt. = 17), PE (and polymers in general) does not have a unique
characteristic molecular weight. One consequence of this is that we have
to always refer to an average molecular weight of the ensemble of polymer
molecules. Two such averages are commonly used. These are
Mn
(g/mol),
5
the number average molecular weight, and
Mw
(g/mol), the weight average
molecular weight. We use bold letters here to indicate the average values.
The definitions of these measures are elaborated below. Typically, the
averagemolecularweightofpolymersincreaseswith
n
(whichistheaverage
degree of polymerization (DP)). For addition polymers such as polyolefins,
the
Mw
(g/mol) increases with DP:
(3.3)
where
N
i
chains have themolecular weight of
M
i
and is themolar fraction (a
numberfraction)
X
i
.However,somepropertiesofpolymersaregovernedby
the fraction of the largest chain molecules in the sample. In such situations,