Chemistry Reference
In-Depth Information
cooperative magnetism due to magnetic interactions (stronger than thermal
energy) between metal ions with unpaired electrons, like in metals, alloys and
simple inorganic compounds (oxides, halides etc.). (2) Molecular magnetism with
weak or vanishing long-range spin-spin interactions, also known as ''molecule-
based magnetism'' [
71
]. The latter kind of magnetism has attracted much interest
in recent years [
72
]. Because of limited space we shall discuss only two examples.
2.3.4.1 Photo-Switchable Prussian Blue Analog
K
0.1
Co
4
[Fe(CN)
6
]
2.7
18 H
2
O
The Prussian-blue analog K
0.1
Co
4
[Fe(CN)
6
]
2.7
18 H
2
O with paramagnetic building
blocks [Co
II
(S = 3/2) -NC - Fe
III
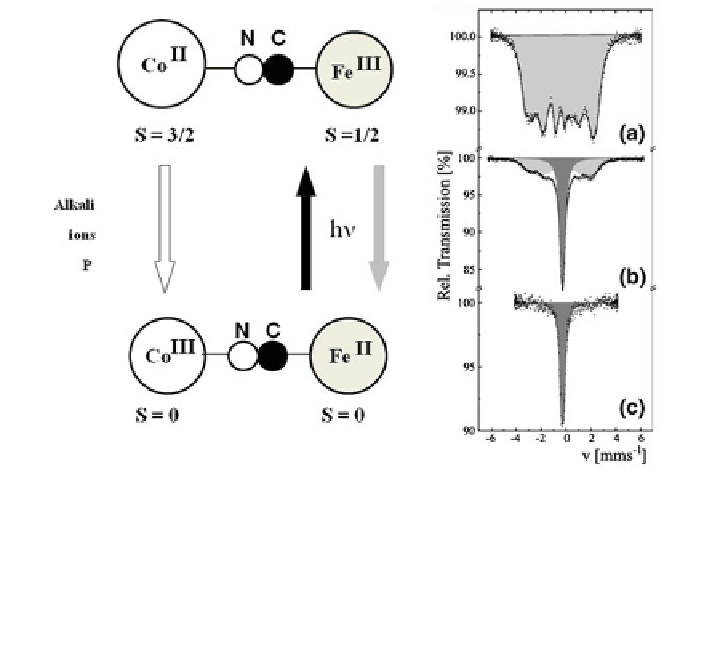
(S = 1/2)] was found to undergo photo-induced
transition by irradiation with blue light, whereby electron transfer takes place to
generate the diamagnetic buildings blocks [Co
III
(S = 0) -NC - Fe
II
(S = 0)]. This
transition is reversible by irradiation with red light (Fig.
2.40
). It was also found that
the transition from Co
II
(S = 3/2) -NC - Fe
III
(S = 1/2)] to [Co
III
(S = 0) -NC -
Fe
II
(S = 0)] is favored by increasing potassium concentration or by replacing
Fig. 2.40 The Prussian-blue analog K
0.1
Co
4
[Fe(CN)
6
]
2.7
18 H
2
O contains photosensitive
paramagnetic [Co
II
(S = 3/2) -NC - Fe
III
(S = 1/2)] building blocks which can be converted
by irradiation with blue light to diamagnetic [Co
III
(S = 0)-NC - Fe
II
(S = 0)] building blocks;
back conversion is possible with red light. Increase of potassium content or replacement of
potassium by bigger caesium ions or application of pressure favors the transition of the
[Co
III
(S = 0) -NC - Fe
II
(S = 0)] entities to [Co
III
(S = 0)-NC - Fe
II
(S = 0)] as confirmed by
Mössbauer spectroscopy at 4.2 K under different pressures a 1 bar, b 3 kbar, and c 4 kbar. Shaded
subspectra correspond to Fe
II
(S = 0) in dark grey and Fe
III
(S = 1/2) in *light grey [
73
]