Chemistry Reference
In-Depth Information
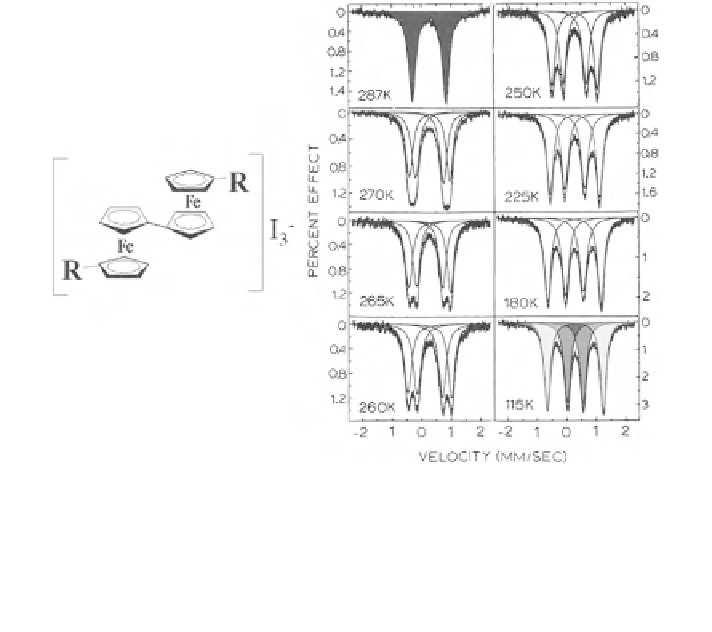
Fig. 2.36 Mixed valence biferrocene with R = Et shows temperature dependent electron
fluctuation between the two iron centers. At low temperatures the fluctuation rate is
comparatively slow (less than the reciprocal of the Mössbauer time window of ca. 100 ns) and
the Mössbauer spectra show two subspectra indicative of ''localized'' ferrocene (grey) and
ferrocinium (dark grey), respectively. At higher temperatures the fluctuation rate becomes so fast
that the Mössbauer spectrum reflects a time-averaged ''delocalized'' species (black) which is
neither ferrocene nor ferrocenium [
66
,
67
]
2.3.3.2 Effect of Crystal Solvents Molecules on the Valence Detrapping
of Mixed-Valence [Fe
3
O(O
2
CCH
3
)
3
)
6
(3-Et-py)
3
]
S
The molecular structure of the mixed-valence compound [Fe
3
O(O
2
CCH
3
)
3
)
6
(3-Et-
py)
3
]
S is visualized in Fig.
2.37
. The molecule accommodates two HS-Fe
III
ions
and one HS-Fe
II
ion which is confirmed by Mössbauer spectroscopy (Fig.
2.37
)
[
68
]. In all spectra the more intense quadrupole doublet (red) corresponds to the
two HS Fe
III
-HS ions and the less intense doublet (green) is for the one HS Fe
II
ion. The ratio of the area fractions of Fe
III
to Fe
II
is close to 2 at low temperatures.
Towards higher temperatures it tends to become larger than 2, which is due to the
larger Lamb-Mössbauer factor of Fe
III
compared to that of Fe
II
. It is found [
68
] that
the mixed valency properties of this compound depend on the nature of the crystal
solvents molecules. Compounds A (with S = 0.5 benzene) and B (with
S = CH
3
CN) appear to be valence-trapped over the whole temperature range up to
room temperature. The quadrupole doublets arising from HS-Fe
III
(red) and HS-
Fe
II
(green) are well resolved and sharp. Thus the lifetimes of these trapped
(localized) species are longer than the lifetime of the 14.4 keV nuclear excited
state. Compound C (with S = CH
3
CCl
3
) are valence-trapped at low temperatures.