Chemistry Reference
In-Depth Information
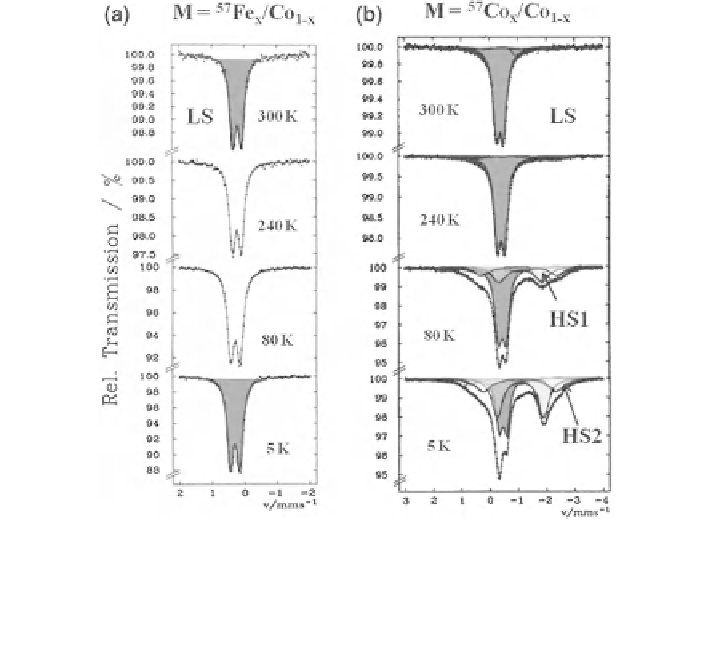
Fig. 2.33 a Left
57
Fe Mössbauer absorption spectra of [
57
Fe
x
/Co
1-x
(phen)
3
](ClO
4
)
2
as a function
of temperature vs.
57
Co/Rh (295 K) as source (x = 0.001) b Right Time-integral
57
Fe Mössbauer
emission spectra of a [
57
Co
x
/Co
1-x
(phen)
3
](ClO
4
)
2
source as a function of temperature vs.
K
4
[Fe(CN)
6
] (295 K) (x = 0.001). In (a) the source was moved relative to the absorber and in
(b) the absorber was moved relative to the fixed source mounted in the crystal [
63
]
As an example, the coordination compound [Co
II
(phen)
3
](ClO
4
)
2
doped with
0.1 % of
57
Fe was studied by Mössbauer absorption spectroscopy using a
57
Co/Rh
source (Fig.
2.33
; spectra on the left). The three phen ligands create a relatively
strong ligand field at the Fe
II
center, the compound shows LS behavior at all
temperatures under study. The same system, however doped with
57
Co as
Mössbauer source, was studied by Mössbauer emission spectroscopy using
K
4
[Fe(CN)
6
] as absorber. The MES spectra (Fig.
2.33
, spectra on the right) also
show the typical Fe
II
-LS signal at 300 K down to ca. 200 K. On further cooling,
however, the intensity of this signal decreases and at the same time two Fe
II
-HS
doublets, HS1 and HS2, appear with increasing intensity [
62
]. These unusual spin
states are excited ligand field states with temperature-dependent lifetimes on the
order of ca. 100 ns.
Similar experiments were carried out with systems whose corresponding Fe
II
compounds possess intermediate ligand field strengths and show thermal spin
crossover. [Fe(phen)
2
(NCS)
2
] undergoes thermal ST as already discussed above
(
Sect. 2.3.2.1
). The temperature dependent MAS spectra are shown on the left of
Fig.
2.34
. The analogous Co
II
compound doped with
57
Co and used as Mössbauer
source (or the corresponding iron compound doped with
57
Co as source which