Chemistry Reference
In-Depth Information
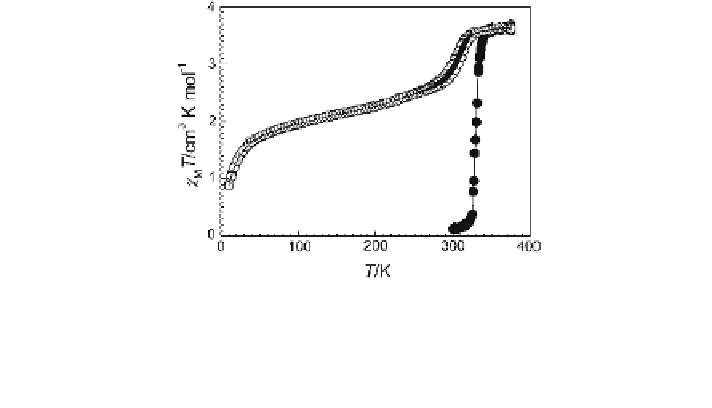
Fig. 2.29 Magnetic properties in the form of v
M
T vs. T of the one-dimensional 1,2,4-triazole-
based mesogen of formula [Fe(C
10
-tba)
3
](4-MeC
6
H
4
SO
3
)
2
nH
2
O, with n = 1(filled circles) and
n = 0(open circles)[
55
,
56
]. The pristine compound is in the LS state at 300 K, but loses crystal
water on heating accompanied by sharp ST to the HS state. The dehydrated sample (n = 0)
shows a entirely different SCO behavior
v
M
T value below 100 K, particularly below 50 K, is due to the ZFS of those iron
atoms which remain in the HS state even at very low temperature, as derived from
the Mössbauer spectrum at 4.2 K (HS population is 48.1 %, LS population is
51.9 %) (Fig.
2.30
c).
Another member of the tba-based mesogen complexes is the room temperature
operational 1D coordination polymer [Fe(C
12
-tba)
3
](CF
3
SO
3
)
2
(C
12
-tba = 3,5-
bis(dodecyloxy)-N-(4H-1,2,4-triazol-4-yl)benzamide) which has been studied by
Mössbauer spectroscopy [
53
]. The disk-like cations are self-assembled in columns,
where the Fe
II
ions are stacked on top of each other in the middle of the column as
sketched in Fig.
2.31
. The distance between the iron ions, determined by powder
X-ray diffraction and Extended X-ray Absorption Fine Structure (EXAFS) mea-
surements, varies with temperature and influences the spin state of the Fe
II
ions as
controlled by Mössbauer spectroscopy (Fig.
2.31
). The variation of the chain
length of the alkoxy substituent on the triazole ligand in [Fe(C
n
-tba)
3
](BF
4
)
2
H
2
O
with C
n
-tba = 5-bis(alkoxy)-N-(4H-1,2,4-triazol-4-yl)benzamide) can dramati-
cally affect the spin state population as illustrated in Fig.
2.32
where the dark grey
and light grey signals refer to the LS and HS states, respectively [
53
].
2.3.2.8 Nuclear Decay-Induced Excited Spin State Trapping (NIESST):
Mössbauer Emission Spectroscopy
In conventional Mössbauer spectroscopy one uses a single-line source, e.g.
57
Co
embedded in a rhodium matrix in the case of
57
Fe spectroscopy, and the iron con-
taining material under study as absorber. This technique is termed Mössbauer
Absorption Spectroscopy (MAS) in order to distinguish it from the so-called source
experiment, also known as Mössbauer Emission Spectroscopy (MES). In a MES