Chemistry Reference
In-Depth Information
k
e
1/s
n
, where s
n
is the lifetime of the 14.4 keV state of
57
Fe. This experiment
was elegantly confirmed a few years later by Maer et al. (Fig.
2.13
b) [
22
].
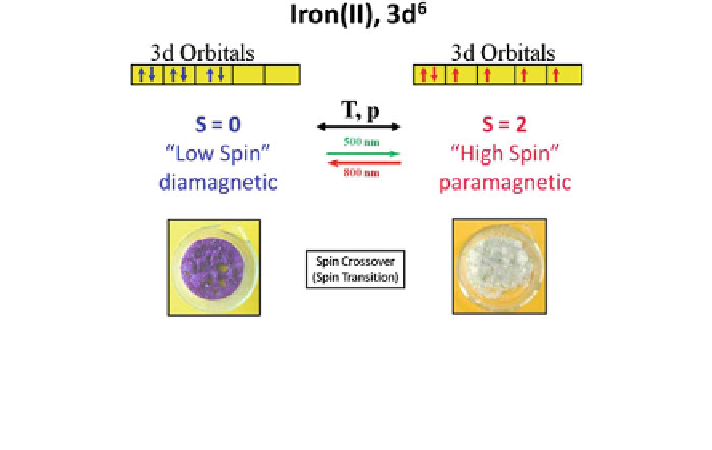
2.3.2 Switchable Molecules: Spin Crossover
2.3.2.1 Thermally Induced Spin State Switching in Fe
II
Compounds
Thermally induced spin state transition from a HS state with maximum unpaired
electrons to a LS state with minimum unpaired electrons can be encountered in
certain transition metal compounds with d
4
up to d
7
electron configurations.
Scheme
2.1
sketches the phenomenon in the case of Fe
II
compounds with six
valence electrons in the 3d shell. In Fe
II
compounds with relatively weak ligands
coordinated to the iron ions, e.g. water molecules, the 3d electrons are accom-
modated spin-free according to Hund's rule of maximum spin of S = 2. Such
compounds, called HS complexes, are paramagnetic and are generally weakly
colored. In Fe
II
compounds with relatively strong ligands like CN
-
ions, the six
electrons are arranged spin-paired with total spin S = 0. Such compounds are
called LS complexes; they are generally diamagnetic and often colored. If the right
kinds of ligands are chosen, e.g. derivatives of tetrazole or triazole, one may
observe spin state transition solely by varying the temperature, applying pressure
or under irradiation with light [
23
-
25
].
Thermally induced spin crossover (SCO) in Fe
II
compounds is reflected by
changes in the electron configuration. In the notation of ligand field theoretical
Scheme 2.1 Classification and physical properties of octahedral Fe
II
coordination compounds.
Most of them (an estimate of [95 %) show either HS or LS behavior depending on the ligand
field strength set up at the metal center by the coordinated ligand molecules. Less than 5 % (about
200 as a rough estimate), mainly those with FeN
6
core, exhibit thermally induced spin crossover.
Spin crossover is also possible by application of pressure and irradiation with light