Chemistry Reference
In-Depth Information
Effect of p-back donation in [Fe(CN)
5
X
n-
]
(3 ? n)-
Table 2.2
zero point, one finds the ordering given in Table
2.2
which expresses the varying
effects of d
p
-p
p
backdonation for the different sixth ligand X.
The isomer shift values become more positive on going from NO
+
to H
2
O. The
reason is that in the same ordering the strength of d
p
-p
p
back donation decreases
causing an increasing d-electron density residing near the iron center and thus
effecting stronger shielding of s-electrons by d-electrons, which finally creates
lower s-electron density at the nucleus in the case of H
2
O as compared to NO
+
.
The fact that the nuclear factor DR/R is negative for
57
Fe explains the increasingly
positive isomer shift values in the given sequence from NO
+
to H
2
O.
2.3.1.4 Effect of Ligand Electronegativity
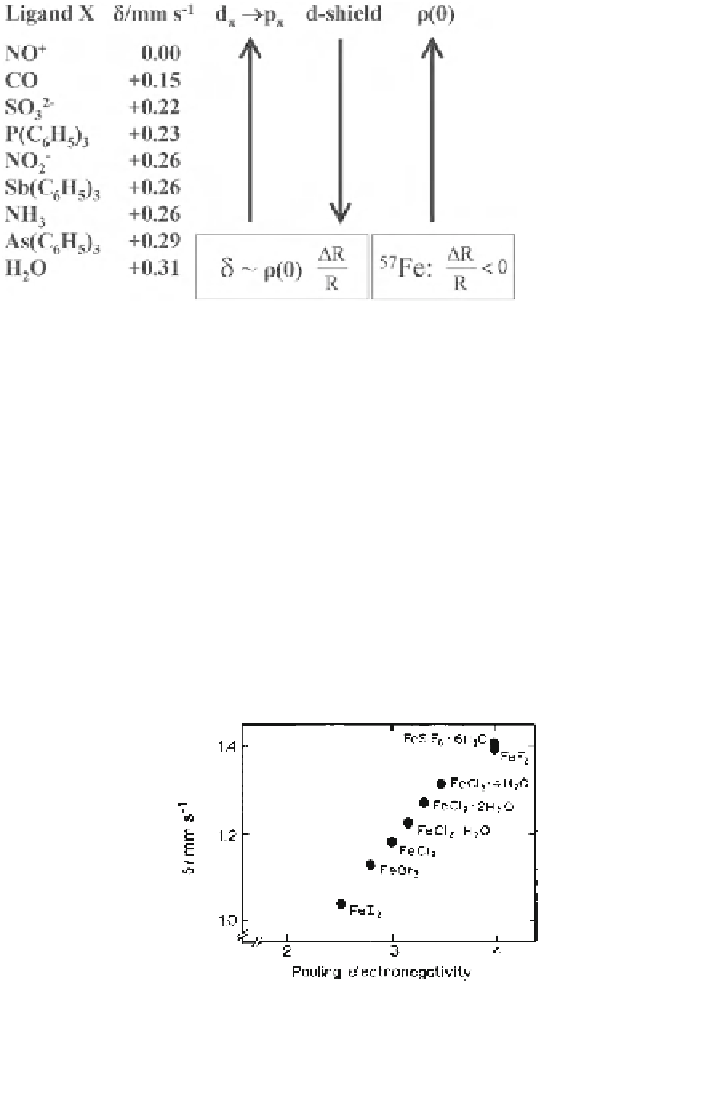
In Fig.
2.12
isomer shift values of ferrous halides taken from the literature [
7
] are
plotted as a function of Pauling electronegativity values. The electronegativity
Fig. 2.12 The graph shows the influence of electronegativity on the isomer shift of ferrous
halides. The electronegativity increases from iodine to fluorine. In the same ordering the 4s
electron population decreases and as a direct consequence the s-electron density at the iron
nucleus decreases, and due to the fact that (R
2
- R
2
) \ 0 for
57
Fe the isomer shift increases from
iodide to fluoride