Chemistry Reference
In-Depth Information
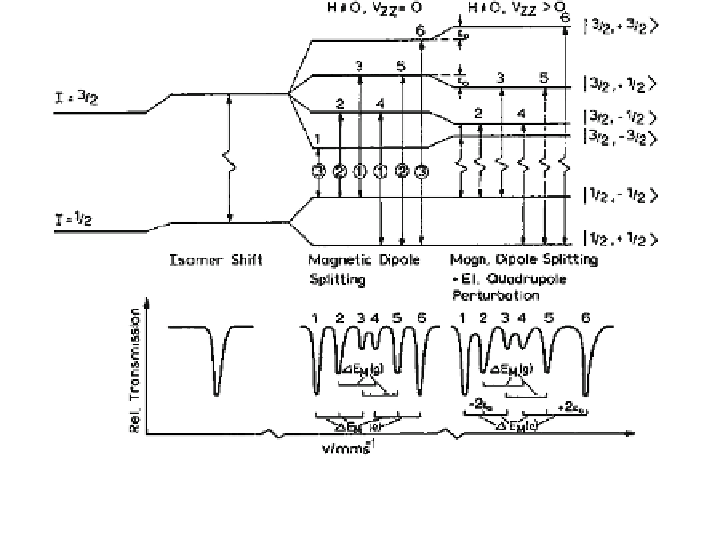
Fig. 2.7 Nuclear energy level scheme (
57
Fe) for electric monopole interaction (causing the
isomer shift, left), pure magnetic dipole interaction (causing magnetic splitting, middle), and
combined magnetic dipole interaction and electric quadrupole interaction (right)
2.3 Selected Applications
2.3.1 Basic Information on Structure and Bonding
2.3.1.1 Quadrupole Splitting in Three Typical Fe
II
Compounds
Figure
2.8
shows the Mössbauer spectra of three selected Fe
II
compounds.
Ferrous sulphate, formulated as [Fe(H
2
O)
6
]SO
4
H
2
O, is a high-spin (HS)
compound with spin S = 2 and shows a large quadrupole splitting of ca.
3mms
-1
.K
4
[Fe(CN)
6
] is a low-spin (LS) compound with S = 0 and cubic (O
h
)
molecular symmetry and shows no quadrupole splitting. Na
2
[Fe(CN)
5
NO] is also
LS with S = 0, but strong tetragonal distortion from O
h
symmetry due to the
replacement of one of the six CN
-
ligands by NO, gives rise to a significant
quadrupole splitting. The occurrence of quadrupole splitting in [Fe(H
2
O)
6
]-
SO
4
H
2
O and Na
2
[Fe(CN)
5
NO]
2H
2
O and the absence of it in K
4
[Fe(CN)
6
] are
explained in Figs.
2.9
and
2.10
.
For HS Fe
II
with 3d
6
electron configuration, the six 3d electrons are distributed
under O
h
symmetry as shown in Fig.
2.9
(left). The two degenerate e.
g
orbitals
carry one electron each, and the three degenerate t
2g
orbitals are occupied by 1
1/3
electrons on average. As the t
2g
and e
g
orbitals are cubic subgroups, there is no
valence electron contribution to the EFG independent of the number of electrons