Chemistry Reference
In-Depth Information
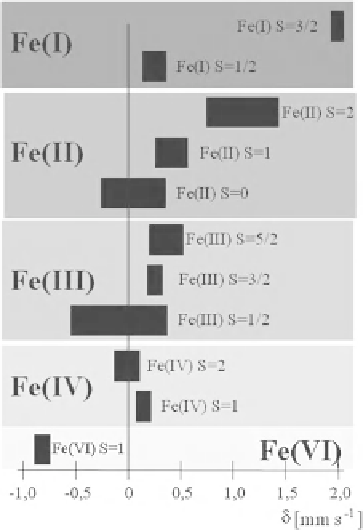
Fig. 2.3 The figure shows ranges of isomer shift values as expected for different oxidation and
spin states. The most positive isomer shift occurs with formally Fe
I
compounds with spin S = 3/
2. In this case, the seven d-electrons exert a very strong shielding of the s-electrons towards the
nuclear charge, this reduces markedly the s-electron density q
A
giving a strongly negative
quantity |W(0)|
2
= |W(0)|
2
. As the nuclear factor (R
2
- R
2
) is negative for
57
Fe, the measured
isomer shift becomes strongly positive. At the other end of the isomer shift scale are strongly
negative values expected for formally Fe
VI
compounds with spin S = 1. The reason is that iron
Fe
VI
compounds have only two d-electrons, the shielding effect for s-electrons is very weak in
this case and the s-electron density q
A
at the nucleus becomes relatively high which—multiplied
by the negative nuclear factor (R
2
- R
2
)—pushes the isomer shift value strongly in a negative
direction. It is seen from the figure that some isomer shift regions do not overlap, e.g. Fe
II
HS
compounds with S = 2 can be easily assigned from a Mössbauer spectrum. In other cases with
more or less strong overlapping d values unambiguous assignment to certain oxidation and spin
states may not be possible. In such cases the quadrupole splitting parameter DE
Q
will be included
in the analysis and leads to a conclusive characterization in most cases
If the electric field gradient (EFG) is non-zero, for instance due to a non-cubic
valence electron distribution and/or non-cubic lattice site symmetry, electric
quadrupole interaction as visualized by the precession of the quadrupole moment
vector about the field gradient axis sets in and splits the degenerate I = 3/2 level
into two substates with magnetic spin quantum numbers m
I
=±3/2 and ±1/2
(Fig.
2.4
). The energy difference between the two substates DE
Q
is observed in the
spectrum as the separation between the two resonance lines. These two resonance
lines in the spectrum refer to the two transitions between the two substates of the