Chemistry Reference
In-Depth Information
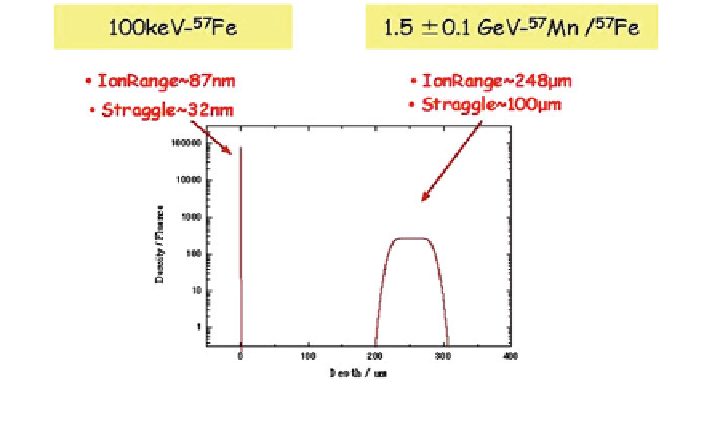
57
Mn/
57
Fe at RIKEN compared with conventional separator
Fig. 6.27
Ion ranges of
matrix during the industrial processes, causing serious degradation in the elec-
tronic properties of silicon-based devices and also solar cells on one hand.
Atomistic information on Fe impurities in Si, on the other hand, is essential to
understand dynamical properties such as atomic diffusion as well as carrier
transport. Fe impurities are known to form the deep levels in the Si band gap,
producing strong trapping centres for the carriers in the devices: Interstitial Fe
i
are
well known to form a donor level at 0.39 eV from the valence band edge, while
substitutional Fe
s
is expected to form an accepter level of 0.69 eV from the first
principle calculation [
45
]. Although interstitial and substitutional Fe have been
detected as spectral components in
57
Fe Mössbauer experiments, the charge states
could not be well distinguished experimentally so far in terms of isomer shifts.
Typical Mössbauer spectra of
57
Fe in n-type FZ-Si in the temperature region
between 330 K and 1,200 K are shown in Fig.
6.28
. The spectra from 300 to 700 K
can be fitted by two singlets of Lorentzians, while the spectra from 800 up to
1,200 K can be analyzed only by a broad singlet. The two singlets at 330 K at the
left and the right hand site have been assigned to
57
Fe atoms on interstitial and
substitutional sites in Si matrix, respectively. This assignment is based on a theo-
retical calculation of the isomer shifts [
46
], which relate with the charge density at
57
Fe nucleus. The spectra and their fitting parameters are changing anomalously
with elevating temperature. These behaviors are in good agreement with the former
experiments on p-type FZ-Si wafers up to 800 K [
39
-
41
]. The resonance area of the
interstitial
57
Fe component at the left hand side decreases strongly above 500 K.
A ''peak position of a component'' in the Doppler velocity scale provides us
information both on the different lattice sites of
57
Fe atoms and their charge states.
This shift is called ''center shift'', depending on the isomer shift and the second-
order Doppler (SOD) shift. As a reference, the broken line in Fig.
6.28
shows the