Chemistry Reference
In-Depth Information
-12
-8
-4
0
4
8
12
-12
-10
-8
-6
V(mm/s)
mm/s
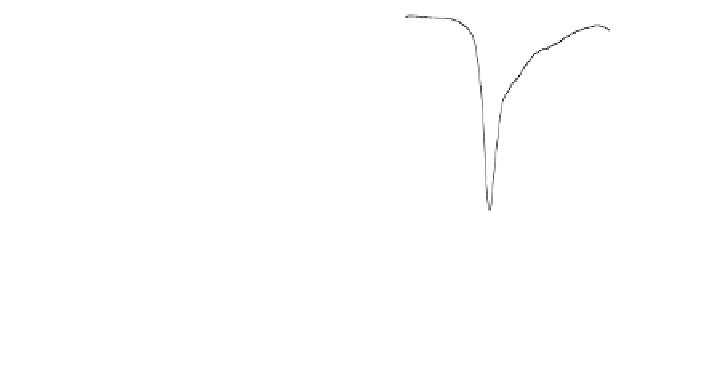
Fig. 4.24 4.2 K Mössbauer spectrum of the 16 h milled ferric fluoride powder and its
decomposition into 3 components (left) and detailed profile of the refinement focused on the low
energy line
as-prepared crystalline state and ground for 0.25, 1, 8 and 16 h. At 300 K, one
observes the reduction of the magnetic component with a progressive asymmet-
rical broadening of lines to the expense of the increase of a single broadened line at
the centre. It is important to emphasize that the application of an external magnetic
field (even rather small produced by a small magnet) favours an increase of the
absorption area of the magnetic component, indicating thus the presence of
dynamics originating from superparamagnetic effects.
At low temperature, the hyperfine structures remain a priori rather independent on
the grinding time but a more detailed analysis of the baseline reveals also an asym-
metrical broadening of sextet lines. These different spectra can be described by means
of discrete distribution of static hyperfine fields, since some magnetic dynamics might
occur at high temperature. At this stage, first remarks are concerned by the values of
isomer shift which are similar to those observed in crystalline and amorphous phases,
andconsequentlyconsistentwithhighspinstateFe
3+
ions (excluding thus the presence
of Fe
2+
) located in FeF
6
octahedral units which are corner-shared.
In the case of the 16 h milled fluoride powder, one can distinguish at 4.2 K from
the static hyperfine field distribution 3 components as illustrated in Fig.
4.24
which
shows the decomposition and the profile of the low energy external line of the sextet
[
165
]. The narrow line sextet is unambiguously attributed to the crystalline AF FeF
3
grains (similar hyperfine field value) while the broad line low field component fairly
compares that of the amorphous FeF
3
phase and is consequently assigned to the grain
boundaries. The hyperfine field characteristics of the third component is found
intermediate between the two previous ones: thus, it does originate from Fe ions
located at the periphery of crystalline grains, giving rise to one Fe atom thick layer
facing the grain boundaries, i.e. one octahedral unit layer, as is schematically rep-
resented in Fig.
4.25
. Such an interfacial layer can be compared to that evidenced in
the case of nanocrystalline alloys (see above section).
When the temperature is increasing, one observes a progressive collapse of the
magnetic sextet into a single line which can be related to the evolution of the