Chemistry Reference
In-Depth Information
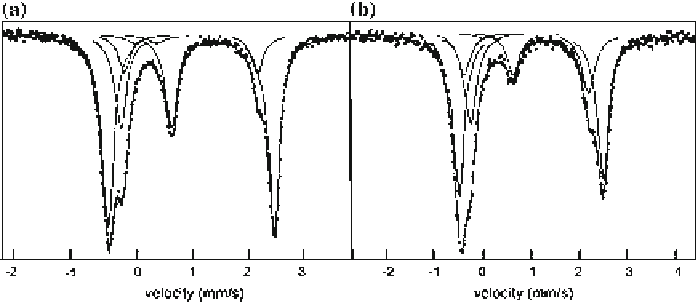
Fig. 3.35
Mössbauer
spectra
at
room
temperature
of
oxidized
vivianites,
Fe
2
þ
3
q
Fe
3
q
ð
PO
4
Þ
2
:
8
q
ð
Þ
H
2
Oq O
ð ;
with (a) q = 0.31 and (b) q = 0.14
3.6.6 Triphylite
Lithium-iron phosphate, LiFePO
4
, has received ample attention in recent years
because of its potential application as electrode active material for rechargeable
lithium batteries (see [
263
] and references therein). It occurs in nature and as such is
known as the mineral triphylite. It has an olivine- type crystallographic structure in
which the ferrous cations occupy strongly distorted corner-sharing octahedral M2
sites, which form zig-zag chains running parallel to the c-axis. A second type of edge-
sharing octahedral sites, M1, forms linear chains that are also directed along the c-
axis and are occupied by lithium cations. Each M1O
6
octahedron shares edges with
two adjacent M1O
6
octahedra, with two M2O
6
octahedra and with two PO
4
tetra-
hedra. The M2O
6
octahedron has common edges with two M1O
6
octahedra and one
PO
4
tetrahedron. Mössbauer spectra of both naturally occurring and synthetic tri-
phylites have been reported by several authors (Van Alboom et al. [
264
] and refer-
ences therein). Below *52 K the material is antiferromagnetically ordered. At
higher temperatures the Mössbauer spectrum consists of a narrow doublet with a
relatively high quadrupole splitting (see Table
3.19
).
3.6.7 Heterosite
This mineral, ideally Fe
3+
PO
4
, possesses the same structure as triphylite, however,
with all M1 sites being vacant. Actually, the two mentioned minerals are the end
members of a complete solid solution. Heterosite also forms a solid solution with
purpurite, MnPO
4
. The Mössbauer spectrum recorded at 80 K for a natural
Mn-substituted heterosite species (Buranga, Rwanda) is reproduced in Fig.
3.36
and very similar spectra were observed for all temperature higher than *60 K