Chemistry Reference
In-Depth Information
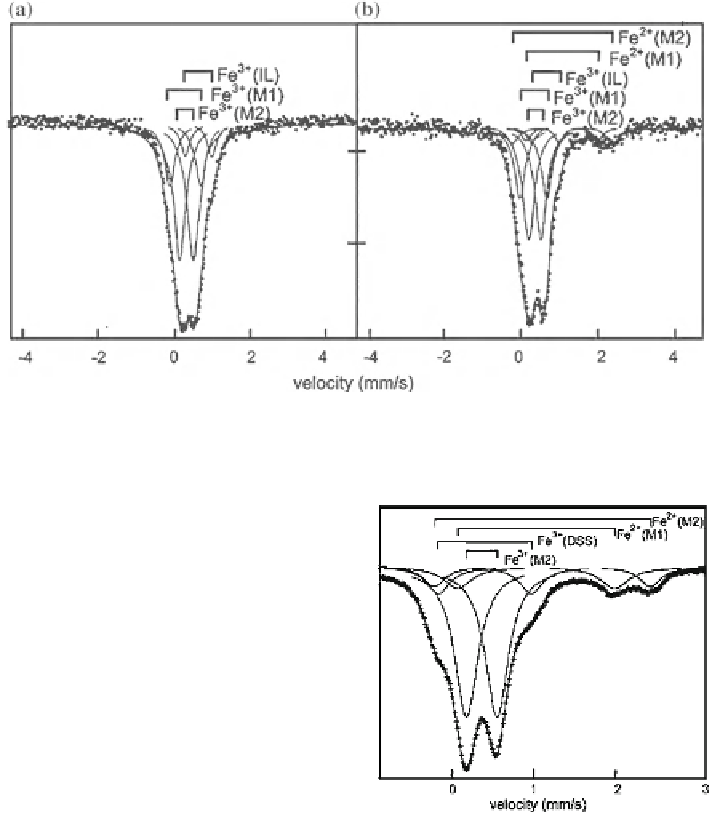
Fig. 3.30 RT spectra of two natural glauconiet samples fitted with three (a) or five doublets
(b) (adapted from De Grave and Geets [
309
])
Fig. 3.31 RT spectrum of a
natural celadonite sample
fitted with four doublets
(adapted from Bowen et al.
[
245
])
doublet (Fig.
3.30
). De Grave et al. [
244
] assigned the latter to interlayer Fe
3+
,
whereas Johnson and Cardile [
241
] analyzed with a third ferric doublet with rather
small D which they attributed to tetrahedral Fe
3+
.
The spectrum of celadonite can be adjusted with two ferrous doublets and one
ferric doublet, the latter assigned to M2 sites (cis) [
245
]. A second weak Fe
3+
doublet with relatively large quadrupole splitting (D & 1.1-1.2 mm/s) (Fig.
3.31
)
has been ascribed to iron in dehydroxylated surface sites (DSS) [
246
,
247
].
The basic mineral of the 2:1 trioctahedral silicates is talc with formula
Mg
3
Si
4
O
10
(OH)
2
. It is obvious that for this silicate Fe
2+
will be the most abundant
valence of iron and consequently the spectra consist predominantly of ferrous
doublets [
246
,
248
]. Similarly, this is also the basic spectral appearance for all