Chemistry Reference
In-Depth Information
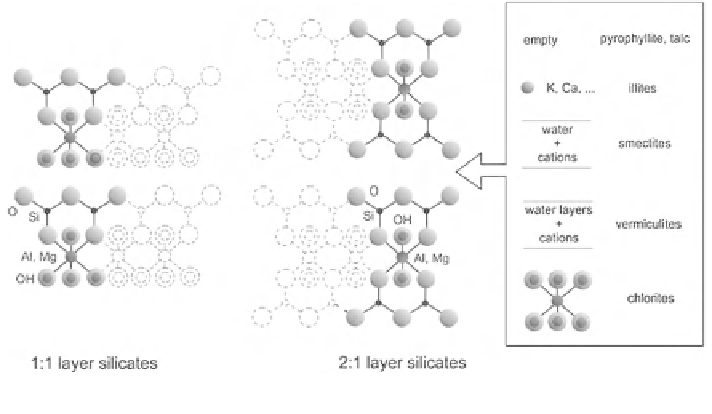
Fig. 3.29
Stacking of octahedra and tetrahedra in phyllosilicates
M1 and M2. The basic blocks may either electrically neutral such as in pyro-
phyllite or talc, but more frequently there is a charge deficiency which must be
compensated. In the micas the substitution of Al
3+
for a quarter of the Si
4+
is
compensated by a 12-fold coordinated large K
+
ion in the interlayer position. In
the so-called brittle micas two silicons are replaced by aluminum and the interlayer
cation is divalent. The repeat distance of the layer is close to 1 nm. The smectites
and vermiculites contain water or water layers with cations in solution. In the
former the repeat distance is variable according to the water content. Because
vermiculites are usually better crystallized the charge deficit is higher which
makes them difficult to expand. Their normal state is a double layer of water giving
the characteristic 1.4 nm spacing. In chlorites the interlayer is occupied by a
brucite (Mg,Al)
3
(OH)
6
sheet which compensates for the charges. The ionic
bonding between the brucite sheet and the talc layers also prevents the chlorites to
expand. Similar to 1:1 layer silicates, 2:1 layer species can be dioctahedral or
trioctahedral.
3.5.6.1 1:1 Layer Silicates
Kaolinite is the basic dioctahedral 1:1 layer silicate. The ideal formula is
Al
2
Si
2
O
5
(OH)
4
. It tends to contain very little iron and it has been shown that ferric
iron can substitute for Al to a very small amount. Murad and Wagner [
234
] sum-
marized the published Mössbauer data giving on the average d
Fe
= 0.34 mm/s and
D = 0.52 mm/s for the ferric doublet. These hyperfine parameters are also repre-
sentative for superparamagnetic iron oxyhydroxides and oxides which are com-
monly associated with kaolinite. Therefore spectra at very low temperatures
(below the blocking temperatures of the oxides) are necessary to distinguish the