Chemistry Reference
In-Depth Information
Table 3.17
Representative hyperfine parameters at RT for some amphiboles
Mineral
Formula
Fe site
d
Fe
(mm/s) D (mm/s)
Fe
2+
Grunerite
Fe
7
Si
8
O
22
(OH)
2
M1
1.16
2.82
Fe
2+
M4
1.10
1.8
(Mg,Fe
2+
,Mn)
7
Si
8
O
22
(OH)
2
Fe
2+
M1-M3
Cummingtonite-
grunerite
1.16
2.81
Fe
2+
M4
1.10
1.5-1.8
(Mg,Fe
2+
)
7
Si
8
O
22
(OH)
2
Fe
2+
M1-M3
Anthophyllite
1.12
2.6
Fe
2+
M4
1.10
1.8
Na(Fe
2+
)
3
(Fe
3+
)
2
Si
8
O
22
(OH)
2
Fe
2+
Riebeckite
M1
1.14
2.83
Fe
2+
M3
1.11
2.32
Fe
3+
M2
0.38
0.43
(Li,Fe
3+
,Mg,Fe
2+
)
7
Si
8
O
22
(OH)
2
Fe
2+
Holmquistite
M1
1.13
2.8
Fe
2+
M3
1.1
2.0
Fe
3+
M2
0.38
0.3
Fe
2+
Ferroactinolite
Ca
2
Fe
5
Si
8
O
22
(OH)
2
M1,M3
1.15
2.81
Fe
2+
M2
1.14
1.85-2.1
(Fe
2+
M4)
1.10
\1.8
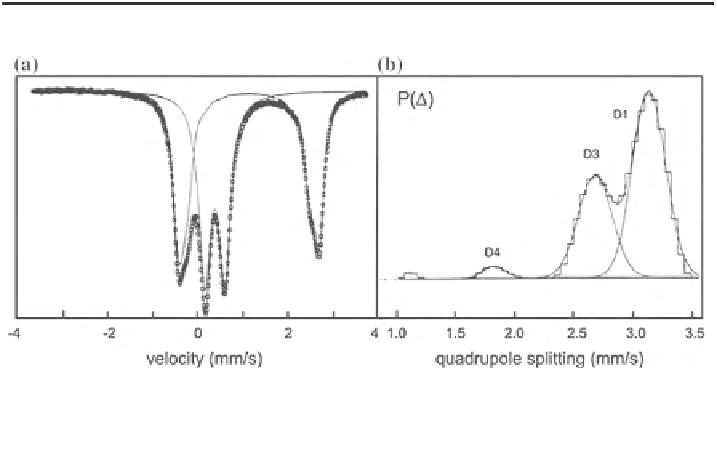
Fig. 3.28 Spectrum at 80 K of a natural riebeckite sample, fitted with quadrupole distributions
(a); resulting quadrupole distribution for Fe
2+
showing three peaks (adapted from Van Alboom
and De Grave [
221
])
It is worth to mention that the analysis of the spectra of pyroxenes has not always
been successful by using discrete doublets. Already in the early days of Mössbauer
investigations of pyroxenes, Bancroft [
3
] argued that the shortcomings of the until-
then commonly applied fitting procedures are the result of the non-uniform chemical
environment for both iron sites requiring rather a set of doublets for each site. The use
of shape-independent quadrupole distributions seem to be more appropriate in that
case [
229
] and has indeed been successfully applied to the analyses of the spectra of
pyroxene minerals such as aluminium diopsides [
230
] and magnesian hedenbergites
[
211
] and of amphiboles such as riebeckites [
231
] (Fig.
3.28
).