Chemistry Reference
In-Depth Information
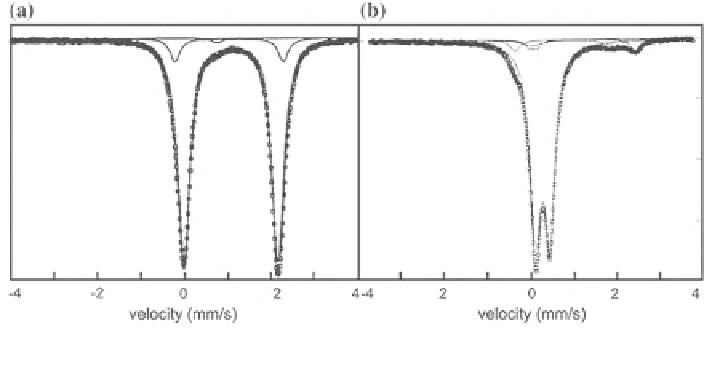
Fig. 3.27
RT spectra of hedenbergite (a) and aegirine (b)
hopping occurs [
197
,
216
] and consequently the quadrupole splitting depends
strongly on the composition.
Beside the clino- and orthopyroxenes there exists a group of minerals with
similar chemical compositions of pyroxenes called pyroxenoids. There structures
are based on twisted chains of silica tetrahedra. An example is rhodonite
(Mn,X)SiO
3
(X = Fe, Ca, Mg), which contains five different sites: three moder-
ately and one strongly distorted octahedral sites and one seven-fold coordinated
site. Spectra of rhodonite and fowlerite (Zn-rich rhodonite) needed to be adjusted
with five doublets showing Fe
2+
to be present in all the available sites [
217
,
218
].
Other minerals, which are structurally similar to pyroxenes except that the
octahedral strips form zigzags, are carpholites, (Mn, Fe
2+
)Al
2
(Si
2
O
6
)(OH)
4
.In
these mineral species the ferrous iron is found to be in a M2 position that is
virtually undistorted. Consequently a very large quadrupole splitting of 3.20 mm/s
is observed [
209
,
210
].
3.5.5.2 Amphiboles
The amphiboles consist structurally of double Si
4
O
11
chains parallel to the
orthorhombic or monoclinic c axis. The general formula of the amphiboles is
W
x
X
2
(Y
5
)(Si
4
O
11
)
2
(OH)
2
in which Y represents the octahedral M1, M2 and M3
sites, X denotes the cation in the irregular M4 site, similar to the M2 site in
pyroxenes, and W is the cation in the ten- to 12-fold coordinated A site which
resembles the interlayer sites in micas. The A site is often empty. Similarly to the
pyroxenes the M4 site can accommodate Ca, Na, Li, Mg and Fe
2+
and the M1-M3
sites are occupied by Fe
2+
, Mg, Fe
3+
, Al, Mn,… The A site, if occupied,
accommodates large monovalent ions such as Na or K. The relative abundances of
the various sites M1:M2:M3:M4:A is 2:2:1:2:x with 0
x
1.