Chemistry Reference
In-Depth Information
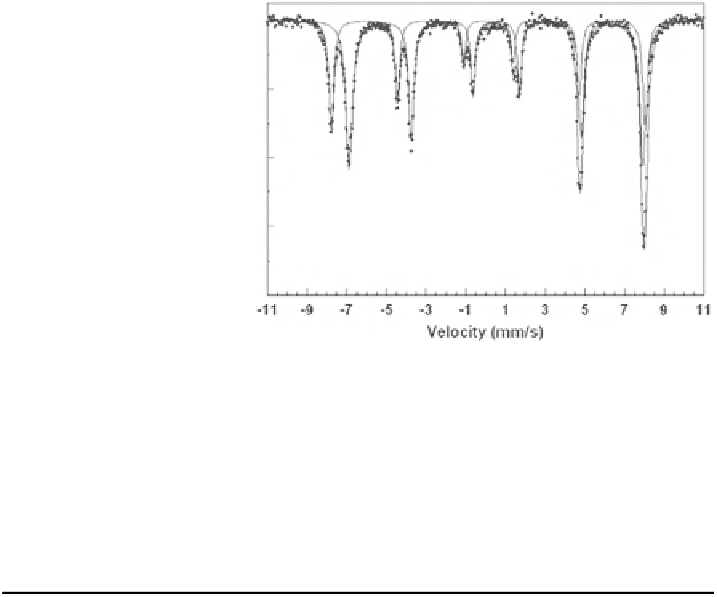
Fig. 3.13 Typical spectrum
of magnetite at RT with outer
Fe
3+
and inner Fe
2.5+
sextet
Table 3.6
Representative hyperfine parameters of magnetite
Magnetite
T (K)
Spectrum
B
av
(T)
d
Fe
(mm/s)
Fe
3+
Pure Fe
3
O
4
RT
A
49.0
0.28
Fe
2.5+
B
45.9
0.66
Fe
3+
130
A
50.4
0.36
Fe
2.5+
B
48.0
0.76
Fe
3+
Oxidized Fe
3-x
O
4
RT
A
49.0
0.28
Fe
3+
B
*50.0
0.36
Fe
2.5+
B
45.9
0.66
often made—but instead should speak about one Fe
3+
sextet and one Fe
2
:
5
þ
sextet
(Table
3.6
).
The observed value for the S(Fe
2.5+
)/S(Fe
3+
) ratio of a magnetite phase can be
used to determine its degree of oxidation. Oxidized magnetite has the general
formula Fe
3-x
O
4
with 0 \ x \ 0.33. In that case one can expect the following
structural formula,
A
½
Fe
2
:
5
þ
2
ð
1
3x
Þ
Fe
3
5x
h
x
B
O
4
;
where h stands for the vacan-
cies and where an equal amount of octahedral Fe
2+
and Fe
3+
results in Fe
2.5+
.
Considering the ratio R = S(Fe
2.5+
)/S(Fe
3+
) to be about 1.8 for pure magnetite at
RT, one can write
Fe
3
þ
R
¼
1
:
81
3x
ð
Þ
ð
3
:
5
Þ
1
þ
5x
for oxidized magnetite, leading to
x
¼
1
:
8
R
5
:
4
þ
5R
ð
3
:
6
Þ
On the other hand, it has been claimed that in the case of oxidation of mag-
netite, the vacancies might be present on both lattice sites [
113
,
114
]. Anyway,