Chemistry Reference
In-Depth Information
p
w
ð
0
Þ
Z
1
g
ð
x
Þ¼
1
dte
þ
i
ð
x
E
0
=
h
Þ
t
e
Ct
=
2h
;
2
ð
1
:
10
Þ
0
or
g
ð
x
Þ¼
w
ð
0
Þ
ih
ð
hx
E
0
Þþ
iC
=
2
;
p
2
ð
1
:
11
Þ
where the decay starts at time t
¼
0 and then the lower limit on the integral can be
set equal to zero. Since E
¼
hx, the probability density P
ð
E
Þ
of finding energy E is
proportional to
j
2
¼
g
ð
x
Þ
g
ð
x
Þ
:
j
g
ð
x
Þ
P
ð
E
Þ¼
const
:
g
ð
x
Þ
g
ð
x
Þ¼
const
:
h
2
2p
w
ð
0
j j
2
ð
hx
E
0
Þ
2
þ
C
2
=
4
:
ð
1
:
12
Þ
The condition
Z
þ1
P
ð
E
Þ
dE
¼
1
ð
1
:
13
Þ
1
yields
C
h
2
w
ð
0
Þ
const
: ¼
j
2
;
j
and P
ð
E
Þ
becomes
P
ð
E
Þ¼
C
2p
1
ð
E
E
0
Þ
2
þð
C
=
2
Þ
2
:
ð
1
:
14
Þ
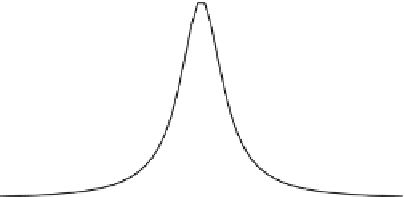
This equation gives the very important fact that the energy of decaying state is
not a constant and is distributed over a region with a width determined by the
decay constant. The width is called natural line width. The shape of the distri-
bution is called a Lorentzian or Breit-Wigner curve as shown in Fig.
1.3
.
Fig. 1.3 Lorentzian
distribution curve of decaying
state. C is the full width of
half maximum
2
/πΓ
I(E)
Γ
1
/πΓ
E
E
0