Chemistry Reference
In-Depth Information
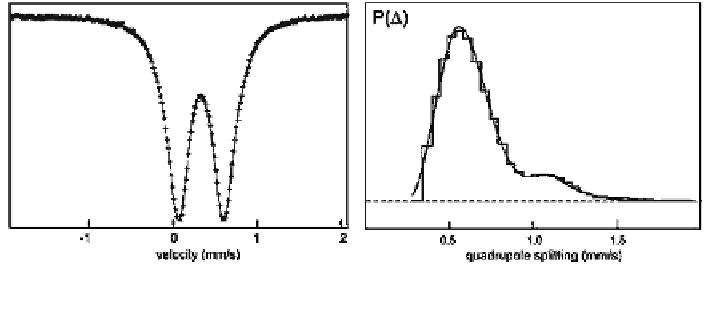
Fig. 3.6 RT Mössbauer spectrum of a lepidocrocite sample (left) and corresponding quadrupole
distribution (right)
3.3.3 Lepidocrocite (c-FeOOH)
The third oxyhydroxide, lepidocrocite, is an antiferromagnet with a magnetic
transition temperature T
N
of about 73 K [
46
]. The magnetic transition is usually
not sharp and exhibits a temperature range, often larger than 10 K, in which a
sextet and a doublet spectrum coexist. Although this would imply a superpara-
magnetic behavior [
47
], similar to other iron oxides and oxyhydroxides, a study of
synthetic lepidocrocites of various particle sizes revealed that surface effects play
in this case a more significant role, leading to a variety of Néel temperatures which
causes the broad transition range [
48
]. This behavior is probably associated with
the typical morphological features of lepidocrocite, which consists of small very
thin, raggedly structured platelets [
47
,
48
].
The RT spectrum exhibits a somewhat broadened doublet (Fig.
3.6
) with an
average quadrupole splitting between 0.55 and 0.7 mm/s, depending on the par-
ticle morphology. A more detailed analysis of the doublet yields quadrupole
splitting distributions which have more or less two maxima (Fig.
3.6
)[
47
]. The
first maximum with D * 0.52 mm/s is attributed to the bulk part whereas the
second with D * 1.1 mm/s is believed to result from the surface species. At 80 K
both distributions are more broadened, so that the second maximum is less pro-
nounced. From diagnostic point of view, these features are not specific enough to
discern lepidocrocite from other oxides or oxyhydroxides and one has to rely on
magnetically split spectra far below 80 K. At 4 K the magnetic spectrum yields a
magnetic hyperfine field of about 44-46 T and a very small quadrupole shift 2e of
about 0.02 mm/s. This hyperfine field is somewhat lower than that of the other iron
oxides and oxyhydroxides, which makes lepidocrocite relatively well discernible
in complex iron oxide samples. However, a distinction between lepidocrocite and
ferrihydrite is much more difficult in view of the broad lines of the latter at low
temperatures. The range of the hyperfine parameter values are given in Table
3.3
.
Similarly to goethite, lepidocrocite can also contain Al [
49
]. From a Mössbauer
study on a series of synthetic Al-substituted samples [
50
] it could be confirmed