Biomedical Engineering Reference
In-Depth Information
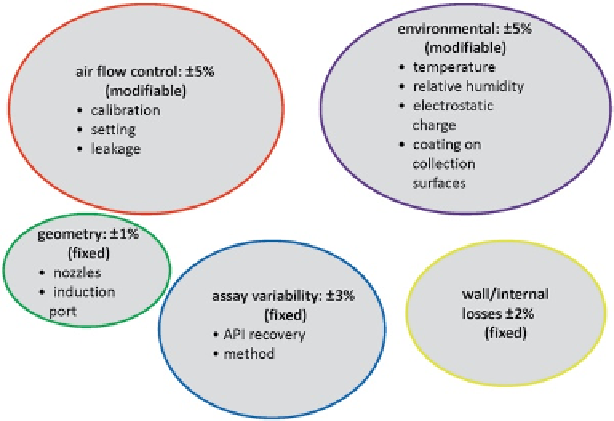
Fig. 4.1
Five principal causes of nonhuman factor-related CI method variability, showing their
contribution to the overall variability of the method (
Adapted from
[
1
]—
used by permission
)
(Fig.
4.1
) are probably unsurprising. Not considering human-related factors, airflow
and laboratory environmental controls were estimated to contribute ±10% of total
variability between them, followed by impactor geometry causes (mainly nozzle
size control) at ±2%, and finally internal losses in the CI providing ±2% to the mea-
sures of OIP performance. CI-related contributors were only part of the overall mea-
surement variability process, with a further ±20% estimated to come from the
inhaler itself in the form of actuation-to-actuation reproducibility through life, as
well as a contribution estimated to be ±3% from the recovery and assay of the API.
4.2
Assessment of Factors Contributing to CI
Measurement Variability
Bonam et al. [
2
], in a comprehensive review of sources of measurement variability
in the CI method, were able to break down the causes of CI method variability in
more detail (Fig.
4.2
). They established that there are four major contributors to
overall variability in CI measurements:
1. Man—the CI operator/analyst
2. Machine—the CI System
3. Measurement—the API recovery and analysis procedure
4. Material—the inhaler including its drug product, including both device and
formulation
Search WWH ::

Custom Search