Biomedical Engineering Reference
In-Depth Information
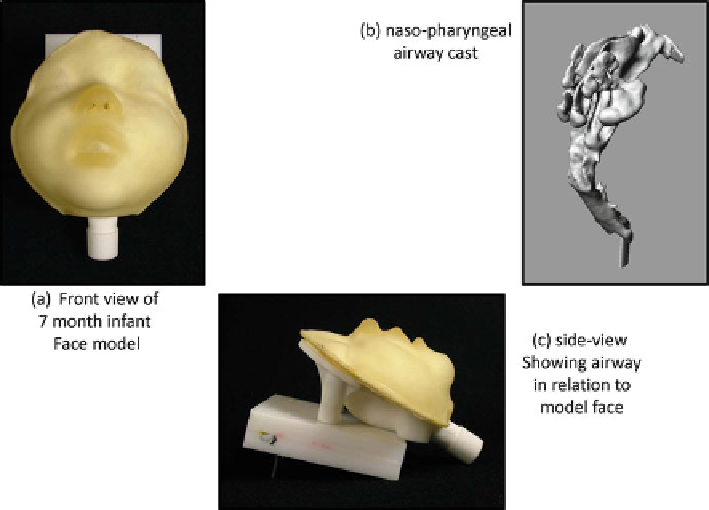
Fig. 12.19
ADAM-III infant face model with anatomically correct nasopharynx (
Facial model
views from
[
61
]
—used with permission, nasopharyngeal model courtesy of W. Finlay
)
This arrangement makes it possible to operate for more than one breathing cycle
(i.e., for the evaluation of devices intended for use by infants or small children, in
which more than one breath may be needed to empty the chamber [
57
]). Use of such
an arrangement could in principle be combined with an AIM-pHRT impactor sys-
tem as well as an idealized or anatomically correct inlet. This type of setup refl ects
the current state of the art with regard to mimicking the patient in the laboratory
setting, and as a result, signifi cant work still needs to be done to validate these
approaches and understand their limitations.
Infants and small children cannot use a mouthpiece as patient interface and so
have to be prescribed inhalers with a facemask [
57
,
58
]. Although studies have been
undertaken to determine in vitro performance of VHC-facemask products with rep-
lica human faces [
59
], there are still no standard models that are commercially
available. Such models should incorporate soft facial tissues to achieve realistic
internal dead space between facemask and face when the facemask is applied with
a clinically appropriate force in the range [
60
]. If they are combined with an ana-
tomically correct upper airway, they are probably the closest that can be currently
obtained to clinical reality in the laboratory [
61
,
62
]. The ADAM-III infant face
was developed at Trudell Medical International, using an anatomically correct
nasopharyngeal upper airway, based on work by Storey-Bischoff and colleagues at
the University of Alberta, Edmonton [
63
]. Figure
12.19
is an example of the state
of the art for this type of modeling.
Search WWH ::

Custom Search