Biomedical Engineering Reference
In-Depth Information
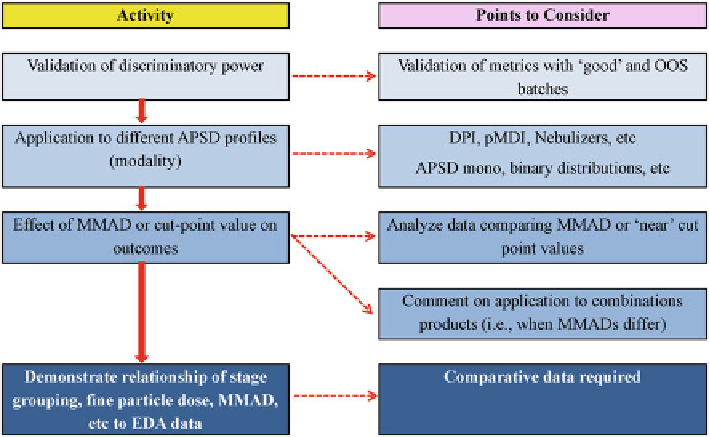
Fig. 11.2
Strategy for EDA to show that this approach is “fi t for purpose”
11.3.3
Strategy for EDA “Fit for Purpose”
The key parts of the strategy to show EDA to be “fi t for purpose” are illustrated in
Fig.
11.2
.
(a) Validation of discriminatory power:
The validation of discriminatory power may form part of the “full-resolution
CI versus AIM comparison” stage of OIP development, when it is considered
that EDA may be the metric of choice (Chap.
6
)
. If an alternate AIM apparatus-
derived metric is being proposed instead of
LPM/SPM
and
ISM
, attention will
be needed to demonstrate discriminating power for small changes in APSD that
is at least as good as current procedures, such as
FPM
<5.0
μ
m
, in Europe or equiv-
alent CI stage groupings in the USA.
(b) Application of EDA to different APSD Profi les arising from single component
and combination products:
It should not be assumed that EDA is going to be applicable to every form of
APSD profi le. Although various product types with differing APSD profi les
were assessed during the initial development of EDA as a concept for general
applicability [
22
,
23
], it is important that an assessment is carried out and that
the requirements needed to meet EDA metrics are met for each new product
under consideration.
One interesting area that has yet to be assessed is that of combination prod-
ucts. Clearly, using AIM to determine “fi ne-particle dose (mass)” would appear
straightforward for both full-resolution CI and even an AIM-based apparatus
Search WWH ::

Custom Search