Biomedical Engineering Reference
In-Depth Information
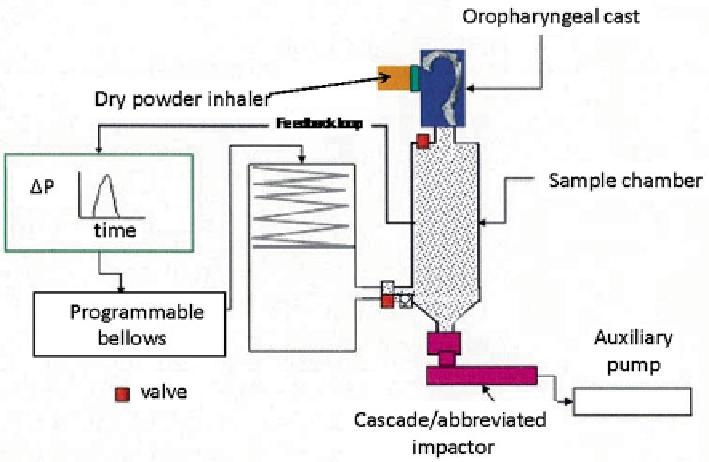
Fig. 10.48
Electronic Lung™ (eLung) set-up for DPI testing by Daniels and Hamilton (
From
[
47
]—
used with permission
)
most likely to be occurring. This group also noted that the testing time to perform
an rNGI measurement and analysis sequence was approximately 2-3 min longer
than that to perform similar operations with the FSI.
To confirm the important observations described earlier, in a further study [
47
],
Daniels and Hamilton investigated the significance of the difference in the ramp-up
phase of the profiles through replication of two patient representative (asthmatic and
COPD) Inhalation profiles using the Electronic Lung™ (eLung) (Fig.
10.48
).
In this work, they sampled a proprietary DPI simultaneously delivering two com-
ponents A and B (Fig.
10.49
) having different
MMAD
values, enabling discrimina-
tion on the basis of
LPM/SPM
ratio with the boundary still fixed at 5.0
m. Values
of
SPM
and
LPM
for either component (Fig.
10.49
) were found to be comparable
between the FSI and full-resolution NGI (note their rNGI was not included in this
comparison).
This outcome was believed to be a result of eliminating any potential for the flow
into the cascade impactor (abbreviated or full resolution) to influence the ramp-up
profile of the DPI and therefore its dose emission characteristics. Separation of the
flow rate profile controlling dose emission to that of dose characterization con-
firmed that the data previously reported by this group [
46
] had arisen as the result of
the difference in ramp-up kinetics between the FSI and NGI.
Both the Pfizer and the initial GSK studies demonstrated the importance of
matching the internal dead volume of an abbreviated CI with that of the full-resolution
CI reference technique for the most accurate results when used in DPI performance
evaluations.
μ
Search WWH ::

Custom Search