Biomedical Engineering Reference
In-Depth Information
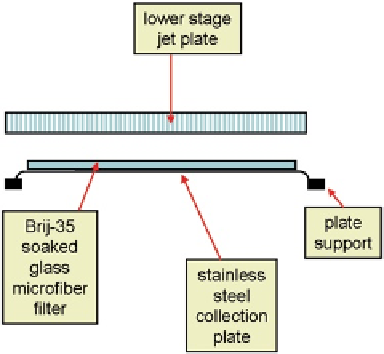
Fig. 10.15
Schematic of
Brij-35-soaked glass
microfiber filter, showing
filter located on top of
stainless steel collection plate
(
From
[
27
]—
used with
permission
)
10.5
Other Studies with ACI-Based AIM Systems
More recently, the commercialized version of the abbreviated ACI (FSA, Copley
Scientific Ltd.) has been extensively evaluated by Keegan and Lewis in connection
with the rapid screening of prototype pMDI actuators in early-stage product devel-
opment [
28
,
29
]. In a rapid prototyping environment, it is often desirable to opti-
mize a device or system based upon the delivered mass and fine particle mass
(
m) that are obtained through the cascade impactor method. A number of
prototypes with slightly altered configurations may be screened to provide an opti-
mized embodiment.
In their first study [
28
], MDIs containing beclomethasone dipropionate (BDP)
(100
≤
5
μ
L) with 13% w/w ethanol cosolvent in HFA134a propellant were man-
ufactured for use as the model product, equipped with a Bespak 630 series actuator
with a 0.22 mm orifice diameter (Bespak, UK). A standard FSA with cut-point sizes
of <5
μ
g/50
μ
m
d
ae
was evaluated as the abbreviated CI configuration. BDP
was recovered only from the impaction plates in the so-called rapid (rFSA) proce-
dure, whereas this API was recovered from all CI surfaces following the standard
method (FSA). The rFSA method therefore permitted as few as three separate actu-
ations from each prototype formulation to be analyzed in terms of
FPM
<5.0
μ
μ
m and <1
μ
m, com-
pared with 4-actuations that were required to achieve the required sensitivity with
the standard (FSA) procedure. The impaction plates were coated after FSA assem-
bly (using either method) using aerosolized 1% w/w glycerol delivered in HFA134a
propellant by a proprietary process. The recovery solvent was an 85:15 methanol-
water mixture.
Following each actuation into the FSA, samples were collected from the USP/Ph.
Eur. induction port and impaction plates (including back-up filter), but interstage
drug loss was not determined. The stack was then reassembled with clean compo-
nents. Following the final actuation, a sample was collected from the actuator and an
average deposition over the appropriate number of pMDI actuations was reported.
Search WWH ::

Custom Search