Biomedical Engineering Reference
In-Depth Information
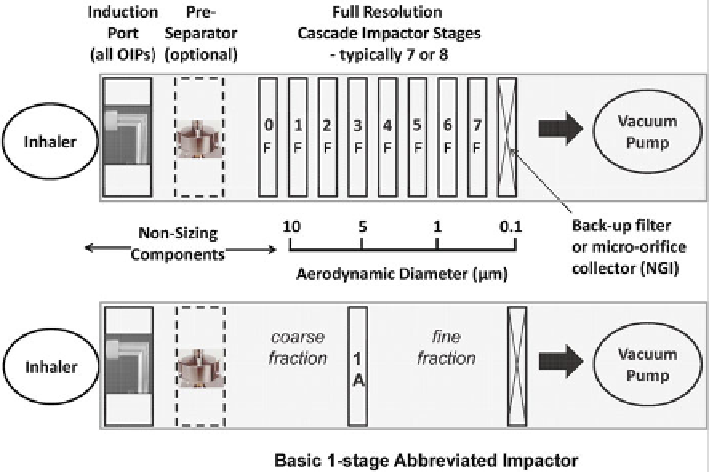
Fig. 1.1
Hypothetical full-resolution CI measurement system (
top
) and a basic 1-stage AIM con-
fi guration having its cut-point close to 5.0
m
d
ae
(
bottom
); the symbols “F” and “A” on the stages
identify full-resolution and abbreviated CI confi gurations, respectively
µ
comparison of the relative size selectivity of individual stages (high) with that of
particle deposition in the various compartments of the HRT (low) [
7
]. In its simplest
form, AIM seeks to reduce the components of the cascade impaction system to the
minimum number required to be able to determine meaningful metrics related to the
cumulative mass of API both fi ner than a certain aerodynamic diameter (
d
ae
) and
larger than this size boundary. Typically, this size boundary is chosen at or close to
5
m to defi ne so-called fi ne and coarse mass fractions in accordance with guidance
provided in the European (Ph. Eur.) [
8
] or the United States (USP) [
9
] Pharmacopeias
(Fig.
1.1
). Although this fi gure depicts the abbreviated and full-resolution CI stage
cut- point (
d
50
) sizes to match, this agreement may not always be necessary (i.e., it
may be appropriate to interpolate data from the full-resolution CI to the stage
d
50
size(s) of the abbreviated system).
AIM-based systems can also include an additional stage with its cut-point size
chosen to be close to 1.0
µ
µ
m
d
ae
for the separate determination of extra-fi ne mass
fraction (Fig.
1.2
).
The coarse mass fraction will likely include the additional API recovered from
the non-sizing components of the impactor system, such as the induction port and
pre-separator (if used). This refi nement in the assessment of the contribution to the
emitted aerosol by coarse particles is essential when evaluating the behavior of
Search WWH ::

Custom Search