Biomedical Engineering Reference
In-Depth Information
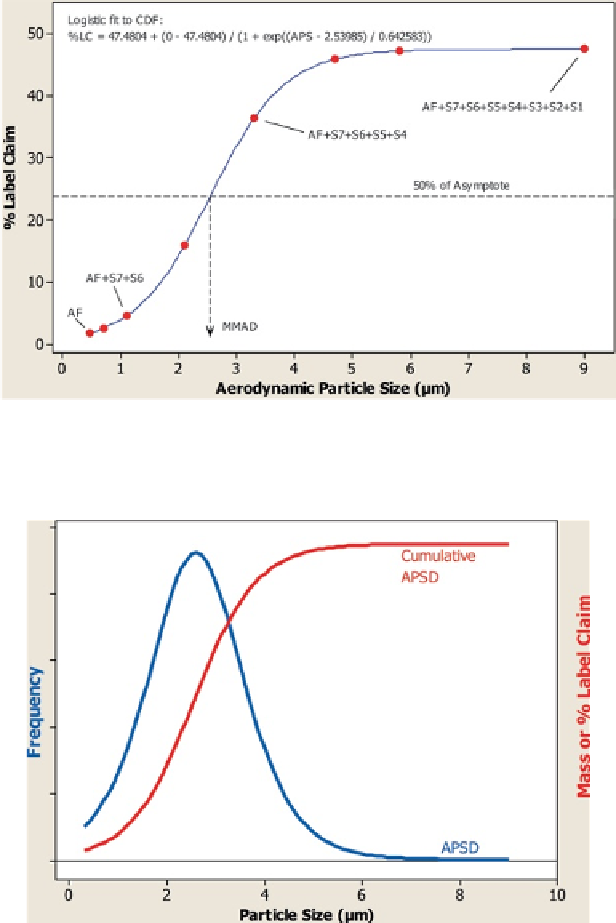
Fig. 7.4
Derivation of the cumulative mass-weighted APSD from the mass API-per-stage results
following a CI measurement
Fig. 7.5
Relationship between the frequency APSD and the cumulative mass-weighted APSD
Internationally the current requirements are varied with respect to the metrics
specified. In the USA, the FDA expects that results from multistage cascade impac-
tors are evaluated based on groupings of the individual stage results [
21
]. Acceptance
criteria are established for each of the 3-4 expected groupings, and samples from a
particular batch must meet the acceptance criteria for each grouping independently
Search WWH ::

Custom Search