Agriculture Reference
In-Depth Information
up transformed plantlets by their ability to fluoresce green when illuminated by
blue light (Eady, 2002; Zheng
et al.
, 2004). The transformation process has been
applied to immature embryos dissected out of developing seeds and cultured along
with the
Agrobacterium
in an appropriate liquid tissue culture medium. These
cultures are a source of cells that can be both transformed and regenerated.
Various groups have developed systems of proliferating cells in culture suitable for
genetic transformation (e.g. Fereol
et al.
, 2005). Dedifferentiated cells termed
'callus cells' are problematic for allium transformation because they can be
genetically unstable and give rise to spontaneous 'somaclonal' genetic variation,
and they can be difficult to regenerate into differentiated plantlets (Novak, 1990;
Eady, 2002).
Using these techniques on several open-pollinated cvs and hybrid parent
lines, onion plants tolerant to herbicides containing glyphosate and phos-
phinothricin (glufosinate) have been engineered and shown to express the genes
and inherit them in a normal Mendelian way (Eady
et al.
, 2003a, b). The plants
grew and bulbed normally (see Fig 3.7a). Such plants were able to withstand
commercial herbicide concentrations, showing that weeds could be controlled
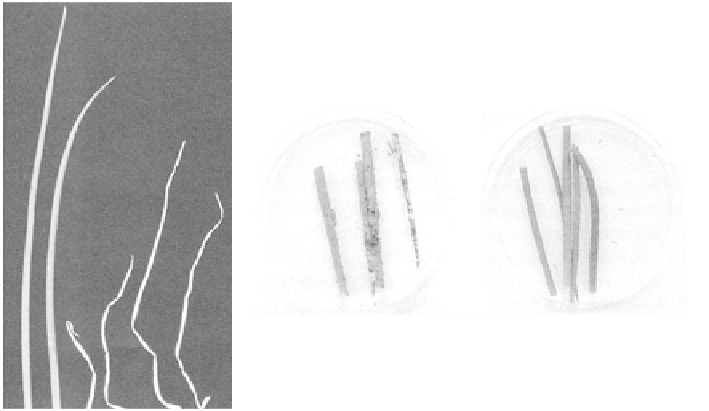
(a)
(b)
Fig. 3.7.
Genetically modified onions. (a) Onion leaves 10 days after spraying with
the contact herbicide phosphinothricin: (left) leaves from a
Bar
-positive plant, a
genetic marker indicating the presence of the herbicide resistance gene; (right)
leaves from non-transgenic plants (from Eady
et al
., 2003b. Courtesy of
Annals of
Applied Biology
). (b) (left) Non-transgenic shallot leaves exposed to the beet army
worm,
Spodoptera exigua
. (right) Leaves similarly exposed from resistant plants
genetically transformed to express an insect-toxic protein coded by the
cry1Ca
gene derived from
Bacillus thuringiensis
(from Zheng
et al
., 2005. Courtesy of
Transgenic Research
).