Environmental Engineering Reference
In-Depth Information
the solution containing 0.1-2
g/mL of MG to 1 g of SDS-loaded iron nanoparticles
in an Erlenmeyer fl ask. The pH of the solutions and iron nanoparticles were sepa-
rately adjusted at 3.0 using 0.1 m/L HCl and/or 0.1 m/L NaOH and the solutions
were stirred for 24 h. The MG loaded nanoparticles were separated with magnetic
decantation. The concentration of MG in the supernatant was monitored spectro-
photometrically by measuring the absorbance of the solution at 627 nm.
ʼ
Results and Discussion
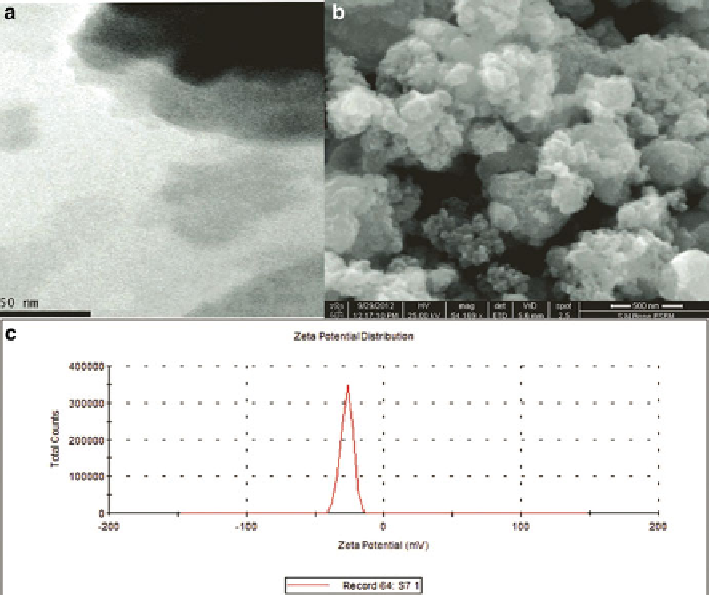
The nanoparticle synthesized in the laboratory was characterized using a dual beam
Techcomp UV2300 UV-vis spectrophotometer. The size of magnetic nanoparticle
was found to be 8 nm by TEM image whereas the scanning electron microscopy
(SEM; S-2300, Hitachi, Japan) revealed amorphous nature of the as-synthesized
iron nanoparticle (Fig.
1
). The DLS data indicated the Zeta potential (
ʶ
) of nanopar-
ticle and was found to be −26.9 mV.
Fig. 1
TEM (
a
), SEM (
b
) images and zeta potential data (
c
) of the synthesized bare magnetic
nanoparticles
Search WWH ::

Custom Search