Biomedical Engineering Reference
In-Depth Information
a
b
25
21
18
15
12
9
6
3
0
15
12
9
6
3
0
20
15
10
10
-1
10
0
10
1
10
2
10
3
10
4
Apparent radius (nm)
10
-1
10
0
10
1
10
2
10
3
10
4
Apparent radius (nm)
5
0
c
d
25
15
12
9
6
3
0
27
24
21
18
15
1
9
3
0
20
15
10
5
10
-1
10
0
10
1
10
2
10
3
10
4
10
-1
10
0
10
1
10
2
10
3
10
4
Apparent radius (nm)
Apparent radius (nm)
0
10
-1
10
0
10
1
10
2
10
3
10
4
10
-1
10
0
10
1
10
2
10
3
10
4
Apparent radius (nm)
Apparent radius (nm)
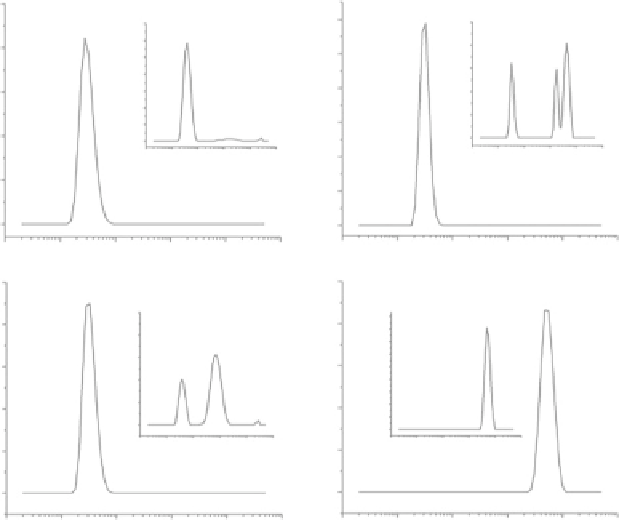
Fig. 5.12 Seeding experiment: N-Hcy-albumin induces aggregation of native unmodified albu-
min. The aggregation state of sample was monitored by DLS. A sample of untreated albumin
(900 μL) is seeded with N-Hcy-albumin (100 μL). Untreated albumin is used as a control. Samples
are incubated at 37
C. Time 0: control experiment (a), seeded sample (c). After 18 days: control
experiment (b), seeded sample (d) (Reproduced from [171])
albumin contains native molecules and only a small amount of large particles
(Fig.
5.12
, panel b) [171].
N-Homocysteinylation initiates albumin aggregation process under physiologi-
cal-like conditions generating large protein complexes formed by native albumin
molecules that precipitate from the solution. The precipitated aggregates, but not
the soluble N-Hcy-albumin monomers, exhibit properties characteristic of amyloid
structures: they become fluorescent when treated with thioflavin T and bind Congo
red, a dye usually used to monitor the formation of amyloid structures. In circular
dichroism (CD) spectral analyses, the aggregates show a reduction of the signals at
both 208 and 222 nm, characteristic of amyloid structures. The aggregation does not
involve disulfide bond formation but hydrophobic interactions facilitated by local
protein unfolding. The early aggregates are cytotoxic and induce apoptosis in
cultured mammalian cells. Furthermore, early aggregates of N-Hcy-albumin can
Search WWH ::

Custom Search