Biomedical Engineering Reference
In-Depth Information
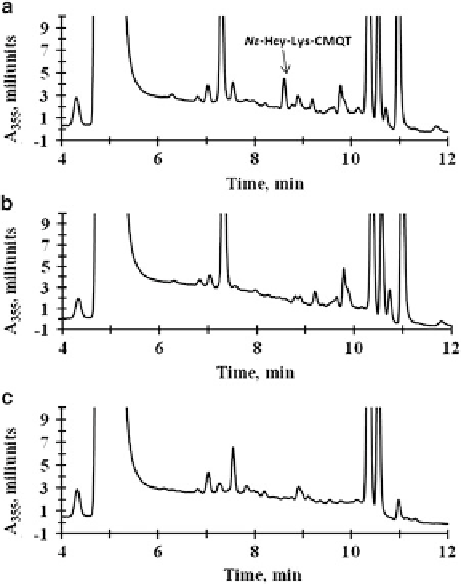
Fig. 5.6 Reversed-phase
HPLC analyses of N-Hcy-
hemoglobin degradation in
mouse liver extracts. Shown
are HPLC traces obtained
with (a), complete reaction
mixture containing N-Hcy-
hemoglobin and mouse liver
extract; (b), N-Hcy-
hemoglobin; and (c), mouse
liver extract. Nε
-Hcy-Lys,
eluting at 8.6 min, is present
only in complete reaction
mixture, indicated by an
arrow in panel (a)
(Reproduced from [72])
mixtures and absent when liver extracts and N-Hcy-hemoglobin are incubated
separately (Fig.
5.6
). The isopeptide Nε
-Hcy-Lys is present in humans and mice,
and its levels increase in hyperhomocysteinemic subjects or mice.
5.3.1.4 Quantification
The Nε
-Hcy-Lys plasma assay [72] is based on the procedure used previously for
the determination of plasma thiols [312]. Plasma (50
μ
L), phosphate buffer (pH 7.4,
0.2 M, 100
μ
L), and tris(2-carboxyethyl)phosphine (TCEP, 0.25 M, 10
μ
L) in
phosphate buffer (pH 7.4, 0.2 M) are incubated for 10 min, and 10
L of 0.1 M
2-chloro-1-methylquinolinium tetrafluoroborate (CMQT) was added. After 3 min,
50
μ
L of 3 M perchloric acid is added to precipitate protein, which was removed by
centrifugation (10 min, 12,000
μ
g). The supernatant is transferred to a vial, and
10
L is injected into a reversed-phase C18 HPLC column (Agilent, Zorbax SB-
C18 4.6
μ
m). The detection and quantification is by UV absorbance at
355 nm. The C18 column separates the Nε
150 mm, 5
μ
-Hcy-Lys-CMQT derivative from CMQT
excess, other aminothiols, and unidentified matrix components. The detection
(LLD) and quantification (LLQ) limits for Nε
M,
respectively. This assay has been used to establish biological significance of
Nε
-Hcy-Lys were 0.08 and 0.1
μ
-Hcy-Lys in humans and mice.
Search WWH ::

Custom Search