Biomedical Engineering Reference
In-Depth Information
160
y = 2.9x - 22.7
R
2
= 0.97
120
80
40
0
0
10
20
30
40
50
60
Number of Lys residues in protein
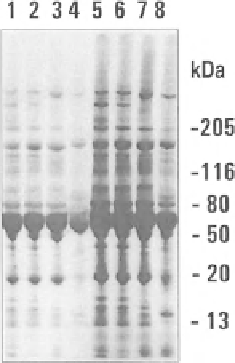
Fig. 5.1 The concentration of a protein and its lysine residues content are major determinants of
N-homocysteinylation. Left panel: Individual proteins present in serum are susceptible to N-
homocysteinylation. Human sera from three donors (lanes 1-3 and 5-7) and rabbit serum (lanes
4 and 8) are incubated with 10 μM (lanes 1-4) or 100 μM (lanes 5-8) [
35
S]Hcy-thiolactone for 4 h
at 37
C and analyzed by SDS-PAGE on 4-20 % gels under reducing conditions. After electro-
phoresis, the gel was stained with Coomassie Blue, dried, and autoradiographed using Kodak
BioMax MR X-ray film. The patterns of Coomassie blue-stained protein bands and
35
S-labeled
bands are identical. An autoradiograph of the gel is shown. Right panel: A relationship between
lysine residues content and protein's reactivity with Hcy-thiolactone. Second-order rate constants
measured at 25
C are plotted as a function of a number of lysine residues per mole of the
following proteins: trypsin, RNase A, DNase I, cytochrome c, myoglobin, MetRS, hemoglobin,
serum albumin, and transferrin (Reproduced from [78])
α
2
-macroglobulin (189 kDa), are the most heavily
35
S labeled [78]. Overall,
each serum protein is N-homocysteinylated proportionally to its abundance
(Fig.
5.1
, left panel).
Individual N-Hcy-proteins are prepared in vitro by incubating a desired protein
with Hcy-thiolactone at physiological pH and temperature. The N-homocystei-
nylation reaction is completed in 4 h at 37
C; the reaction is ten times slower at
25
C [78, 139]. Second-order rate constants for reactions of Hcy-thiolactone with
individual purified proteins are listed in Table
3.3
.
The number of lysine residues is the major determinant of protein's reactivity
toward Hcy-thiolactone. For proteins that vary in size from 104 to 698 amino acid
residues, there is a very good correlation (r ¼
and
0.97) between protein's lysine
content and its reactivity with Hcy-thiolactone (Fig.
5.1
, right panel) [78, 139].
Larger proteins, such as fibrinogen (3,588 amino acid residues) and low-density
lipoproteins (LDL) (~5,000 amino acid residues), react with Hcy-thiolactone ~six-
fold less efficiently than expected from their lysine contents (Table
3.3
), suggesting
great differences in lysine residues reactivities, possibly due to their different
exposures to the solvent. Indeed, subsequent mass spectroscopic studies show
that protein lysine residues exhibit different reactivities toward Hcy-thiolactone.























Search WWH ::

Custom Search