Biomedical Engineering Reference
In-Depth Information
Table 3.4 Second-order rate constants, k, for reactions of Hcy-thiolactone with aldehydes
Aldehyde
k at 25
C(M
1
h
1
)
Formaldehyde
a
8,648
Acetaldehyde
a
2,496
Streptomycin
b
1,200 (2,000 at 37
C)
a
Data recalculated from [84]. Nonlinear kinetics observed suggest the formation of an intermediate
b
H. Jakubowski, unpublished data
R
R
CH
CH
N
HN
O
R
O
CH
O
S
NH
2
S
NH
S
CHR(OH)
2
H
2
O
OH
R
O
R
S
OH
O
CH
CH
S
HN
H
HN
O
O
O
R = H, CH
3
O
S
Reaction 3.5 The mechanism for the formation of 1,4-tetrahydrothiazine form Hcy-thiolactone
and formaldehyde (R¼H) or acetaldehyde (R¼CH
3
). The initial product of the reaction of Hcy-
thiolactone with aldehydes is carbinolamine in a chemical equilibrium with imine. The formation
of the carbinolamine greatly destabilizes the thioester bond by facilitating anchimeric assistance
by the carbinolamine group, which makes possible an intramolecular attack of the oxygen on the
thioester bond to form a five-membered lactone. This leads to the liberation of the thiolate group.
Subsequent attack of the thiolate on an aldehyde-derived carbon leads to rapid formation of the
six-membered ring of the tetrahydrothiazine and lysis of the lactone (Reprinted from [84])
metabolically inactive tetrahydrothiazine via the kidneys. This possibility is
supported by findings showing that acetaldehyde [217] and other aldehydes are
formed in the human body [218] and that in rats injected intraperitoneally with
radiolabeled 1,3-tetrahydrothiazine-4-carboxylic acid, 55 % of the administered
compound is excreted in the urine and only 6 % is expired as carbon dioxide [219].
However, to what extent the formation of tetrahydrothiazines contributes to meta-
bolic flows of sulfur-containing amino acids in animals and humans remains to be
examined.
The reaction of Hcy-thiolactone with o-phthalaldehyde (OPA), generating a
fluorescent adduct, is exploited in sensitive assays for Hcy-thiolactone quantifica-
tion. For example, Hcy-thiolactone separated by HPLC on a cation exchange
column is quantified by monitoring fluorescence after post-column derivatization
with OPA/NaOH [93-95]. An alternative method involves HPLC on a reversed-
phase C18 column, on-column derivatization using an OPA-containing solvent, and
quantification by fluorescence [220] (see the following section).




























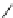
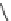

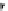








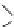






























Search WWH ::

Custom Search