Biomedical Engineering Reference
In-Depth Information
Table 3.3 Second-order rate constants, k, for reactions of Hcy-thiolactone with proteins and
lysine derivatives (pH 7.4)
Protein (kDa) or lysine derivative
k at 25
C(M
1
h
1
)
k at 37
C(M
1
h
1
)
α
2
-Macroglobulin (725)
400
Low-density lipoprotein (500)
150
Fibrinogen (340)
101
γ
-Globulin (140)
112
Transferrin (80)
150
560
Albumin (68)
128
466
Hemoglobin (64)
84
600
MetRS (64)
60
α-Crystallin (36)
10
DNase I (37)
9
Trypsin (24)
9
Myoglobin (16)
40
Cytochrome c (12.5)
36
150
RNase A (12.5)
3
Poly-Lys (150)
6,700
LysLys
26
LysAla
3
Lysine
1
5
α
-N-acetyl-lysine
3.8
ε
-N-acetyl-lysine 1.2
Linear kinetics observed in a wide range of reagent concentrations (compiled from [78, 84])
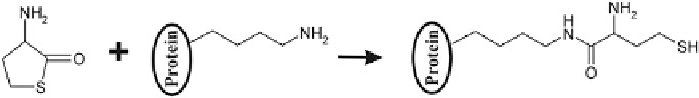
Reaction 3.4 Chemical modification of a protein lysine residue by Hcy-thiolactone (Reprinted
from [68])
temperature 37
C, Hcy-thiolactone modifies proteins by forming N-Hcy-protein
adducts, in which Hcy is N-linked to the
-amino group of protein lysine residues as
shown in Reaction
3.4
[68, 73, 78]. Other amino acid side chain groups in protein do
not appreciably react with Hcy-thiolactone. Indeed, N-linked Hcy is found in
albumin [78, 96, 212, 213], hemoglobin [214], fibrinogen [116, 215], and cyto-
chrome c [136] only on lysine residues. In proteins, the reactivity of side chain
lysine ε-amino residues is much greater than the reactivity of the N-terminal amino
group [78]. In free lysine, the ε-amino group exhibits threefold greater reactivity
with Hcy-thiolactone than the
ε
-amino group of lysine (second-order rate constants,
k
obs
/[Lys], are 3.8 M
1
h
1
and 1.2 M
1
h
1
, respectively, at pH 7.4; Table
3.3
).
These studies were facilitated by the availability of radiolabeled [
35
S]Hcy-
thiolactone of high specific activity [78], prepared as illustrated in Fig.
3.2
.
α


Search WWH ::

Custom Search