Biomedical Engineering Reference
In-Depth Information
a
b
4.2
5
4
3.8
3
3.4
2
1
3.0
56789 0 1
5
6
7
8
9
10
11
pH
pH
c
d
4
10
Formaldehyde
Acetaldehyde
3
9
2
8
1
0
7
5
6
7
8
9
10
11
5
6
7
8
9
10
11
pH
pH
Fig. 3.3 Effects of pH on reactions of Hcy-thiolactone with electrophiles and nucleophiles. Panel
(a), pH dependence of the second-order rate constant for the hydrolysis of Hcy-thiolactone
(k ¼ k
obs
[OH
]
1
). Panel (b), pH dependence of the pseudo-first-order rate constant for the
reaction of Hcy-thiolactone with lysine (k ¼ k
obs
[Lys base]
1
). Panel (c), pH dependence of the
pseudo-first-order rate constant for the reaction of Hcy-thiolactone with human serum albumin
(k ¼ k
obs
[Albumin]
1
). Panel (d): pH dependences of the third-order rate constants (k ¼
k
obs
[OH
]
1
[aldehyde]
1
) of the reactions of HTL with formaldehyde (open circle) and acetalde-
hyde (open square). The solid lines in each panel are theoretical lines for the reactions of acid and
base Hcy-thiolactone forms calculated from the equation k ¼ (k
HTL
+
)(1α)+(k
HTL
0
)α, where α
is the fraction of Hcy-thiolactone in the base form, calculated at indicated pH using a pK
a
¼ 6.67.
Panels (a) and (d)—reproduced with permission from [84]; panels (b) and (c)—H. Jakubowski,
unpublished data
Table 3.2 Apparent rate constants, k, for reactions of base (HTL
0
) and acid (HTL
+
) forms of Hcy-
thiolactone with aldehydes, hydroxide anion [84], free lysine base, and human serum albumin
(H. Jakubowski, unpublished data)
Reactants
k
HTL
0
k
HTL
+
HCHO, OH
10
6
M
2
min
1
10
9
M
2
min
1
44
3.5
CH
3
CHO, OH
11 10
6
M
2
min
1
0.8 10
9
M
2
min
1
OH
37 M
1
min
1
6.9 10
3
M
1
min
1
1,500 M
1
min
1
11,900 M
1
min
1
Lysine base
765 M
1
min
1
Kinetics of the reactions with lysine and albumin are linear, whereas nonlinear kinetics are
observed for the reactions with aldehydes
4 M
1
min
1
Albumin




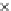


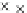


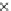

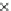




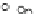
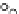
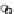

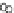
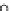














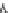

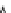
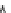
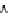






















































Search WWH ::

Custom Search