Biomedical Engineering Reference
In-Depth Information
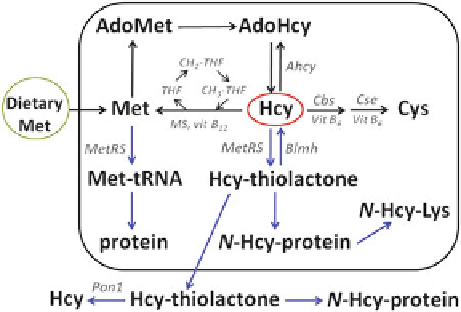
Fig. 1.1 Schematic
representation of human
homocysteine metabolism
its close structural similarity to the coded amino acid methionine, Hcy does enter
the first step of protein biosynthesis. In fact, the active site of methionyl-tRNA
synthetase (MetRS), in addition to activating the cognate substrate methionine and
forming Met-AMP, also activates Hcy and forms homocysteinyl adenylate (Hcy-
AMP). Met-AMP proceeds to form Met-tRNA, which then donates Met to growing
peptide chains in the ribosomal protein biosynthetic apparatus. In contrast, the Hcy-
AMP intermediate is converted to Hcy-thiolactone by an editing mechanism of
MetRS, which prevents the attachment of Hcy to tRNA
Met
and thus precludes Hcy
from entering the genetic code [6, 7]. The editing mechanism of MetRS is universal
and is responsible for the biosynthesis of the thioester Hcy-thiolactone in all
organisms, from bacteria to human. The conversion to Hcy-thiolactone, catalyzed
by MetRS, initiates a novel pathway of Hcy metabolism in humans and animals
(Fig.
1.1
).
Methionine, one of the eight essential amino acids that must be provided in the
form of dietary protein, is the only source of Hcy in the human body (Fig.
1.1
).
Under normal circumstances, Hcy does not accumulate because it is metabolized to
methionine and cysteine. However, the inability to metabolize Hcy via the classical
remethylation and transsulfuration pathways, caused by certain genetic or
nutritional deficiencies, leads to Hcy accumulation, which is associated with
numerous pathological conditions in humans. Over the past two decades, studies
of Hcy have experienced unprecedented growth fueled primarily by the desire to
understand its role in cardiovascular and neurological diseases in humans [8-10].
Early studies have shown that high-protein diets can be harmful to experimental
animals. Subsequent studies of individual dietary amino acids have led to the
conclusion that methionine, the ultimate metabolic precursor of Hcy (Fig.
1.1
),
ingested in excess, is the most toxic amino acid [11-13]. Excessive consumption of
methionine leads to elevation of Hcy levels (hyperhomocysteinemia). In fact, animals
fed with high-methionine diets are often used as models of experimental hyperhomo-
cysteinemia [14]. For instance, female rats fed with a diet containing 5 % methionine
have no successful pregnancies [15], while animals fed with high-protein or high-
methionine diets for 2 years develop hyperhomocysteinemia and vascular disease
[16, 17]. Apolipoprotein E-deficient mice fed with a high-methionine diet (2.2 % or
Search WWH ::

Custom Search