Biomedical Engineering Reference
In-Depth Information
A
A
S
-cysteinylated TTR
13880
S
-cysteinylated TTR
13880
S
-sulfonated TTR
13841
100
100
In vivo
20.7
µ
M homocysteine
In vitro
S
-homocysteinylated TTR
13895
S
-CysGly TTR
13937
unmodified TTR
13761
0
µ
M homocysteine
S
-homocysteinylated TTR
13894
50
50
S
-CysGly TTR
13937
S
-sulfonated TTR
13841
S
-glutathionylated TTR
14067
unmodified TTR
13760
S
-glutathionylated TTR
14066
0
0
B
B
13841
13894
100
100
In vivo
In vitro
434
µ
M homocysteine
500
µ
M homocysteine
13880
50
13895
50
13761
13938
14068
13880
13841
13937
14066
13760
0
0
13700 13750
13800
13850
13900
13950
14000
14050
14100 14150
14200
13700 13750
13800
13850
13900
13950
14000
14050
14100
14150
14200
Molecular mass (Da)
Molecular mass (Da)
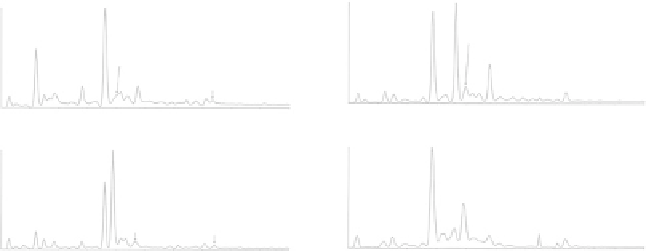
Fig. 7.2 Mass spectrometric analysis of transthyretin S-homocysteinylation in vitro (left panel)
and in vivo (right panel). The transthyretin was immunoprecipitated from human plasma, purified
by reversed-phase HPLC, and analyzed by ESI-MS (Reproduced from [105])
The single Cys
10
residue of TTR carries S-linked Hcy, in addition to cysteine,
glutathione, CysGly, and sulfonyl residue [104, 105]. Low levels of S-Hcy-TTR,
comprising a few percent of total TTR, are detectable by LC-MS in normal human
plasma (Fig.
7.2
). The low abundance of S-Hcy-TTR probably explains why it was
not detected in early studies [416]. In plasma from CBS-deficient and cblC/D-
deficient patients, S-Hcy-TTR levels increase about tenfold to comprise about 20 %
of total TTR (Fig.
7.2
). TTR in human plasma, as well as purified TTR, is also able
to bind Hcy in vitro [105]. Higher fraction of S-conjugated TTR is observed in
patients with symptomatic amyloid disease [417].
Homotetrameric TTR variants, including S-Cys-TTR, S-GSH-TTR, S-CysGly-
TTR-, and S-sulfo-TTR, chemically synthesized starting with wild-type TTR, are
more amyloidogenic than unconjugated wild-type TTR at the higher end of the
acidic pH range (pH 4.4-5.0) [418]. The variants are similarly destabilized relative
to wild-type TTR toward urea denaturation and exhibit rates of urea-mediated
tetramer dissociation (pH 7) and methanol-facilitated fibril formation similar to
those of wild-type TTR. Under mildly acidic conditions (pH 4.8), the
amyloidogenesis rates of the mixed disulfide TTR variants are much faster than
the wild-type rate. S-Sulfo-TTR is less amyloidogenic and forms fibrils more
slowly than wild type under acidic conditions, yet it exhibits stability and rates of
tetramer dissociation similar to those of wild-type TTR when subjected to urea
denaturation.
Conversion of the Cys
10
thiol group to a mixed disulfide with the amino acid
Cys, the CysGly peptide, or GSH increases amyloidogenicity and the
amyloidogenesis rate above pH 4.6, conditions under which TTR probably forms
fibrils in humans. Hence, these modifications may play an important role in human
amyloidosis [418].
The most prevalent modification, S-cysteinylation, renders TTR substantially
more amyloidogenic than wild-type TTR at pH 5, which may be a risk factor for the
Search WWH ::

Custom Search