Biomedical Engineering Reference
In-Depth Information
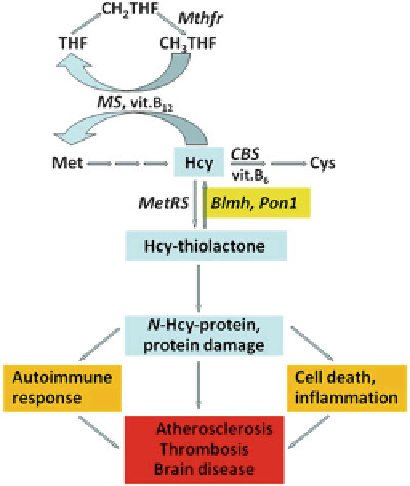
Fig. 6.1 The pathology of
hyperhomocysteinemia: the
Hcy-thiolactone hypothesis
(Adapted from [93])
observed in cardiovascular and neurodegenerative diseases [69, 73, 74, 78, 134].
The thioester chemistry of Hcy-thiolactone underlies its ability to form stable
isopeptide bonds with protein lysine residues, which impairs or alters the protein's
function. Major pathophysiologic consequences linked to the accumulation of N-
Hcy-protein include inflammation [169], cell death [170, 171], an autoimmune
response [134, 135, 172, 173], and interference with blood clotting (Fig.
6.1
)
[174, 175].
The verification of Hcy-thiolactone hypothesis became possible with the devel-
opment of sensitive chemical [79] and immunological assays [168, 357] for detec-
tion and quantification of Hcy-thiolactone [94, 95] and protein N-linked Hcy in
humans [115, 297] and mice [93, 113]. Evidence supporting the Hcy-thiolactone
hypothesis is discussed in a greater detail in the following sections.
6.1.1 Hcy-Thiolactone Levels in Hyperhomocysteinemia
The Hcy-thiolactone hypothesis predicts that Hcy-thiolactone will be elevated
under conditions predisposing to cardiovascular or neurodegenerative disease,
such as hyperhomocysteinemia due to mutations in genes encoding Hcy or folate-
metabolizing enzymes. This prediction has been confirmed in vivo in humans and
mice. For example, CBS-deficient patients have greatly elevated Hcy-thiolactone
levels [93]: mean plasma Hcy-thiolactone concentration in CBS
/
patients
(14.4 nM) is 72-fold higher than in unaffected CBS
+/+
individuals (Table
3.12
).
Search WWH ::

Custom Search