Agriculture Reference
In-Depth Information
especially useful for loosening and adding CEC to heavy low-CEC clay soils such
as the kaolin clays common in the tropics and subtropics. They may also be put to
good use in potting mixes.
Natural zeolites form where fresh volcanic lava reacts with sea water, and where
volcanic ash layers react with alkaline groundwater. Zeolites also may form in
shallow marine basins. What makes a zeolite a zeolite is its very regular, rigid,
open latticework crystal atomic structure with a network of interconnected pores.
Zeolites may have a surface area of up to 450 meters
2
/gram and as much as 45%
open pore space for holding water and exchangeable cations.
1
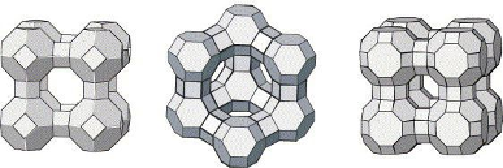
. Crystal structure of zeolites.
Note the pyramidal structure of
Clinoptilolite, center.
Exchangeable Minerals in Zeolites
Zeolites that form from volcanic lava in contact with sea water, and those formed
in other saline environments, may have a high content of Sodium, though often
this has been displaced over time, largely by Potassium. One should know the
typical mineral analysis of any zeolite they are using in agriculture.
Restoring Aged Clays
In some cases the soil may have a higher potential exchange capacity, but the
clays it containsconsist of collapsed clay layers that have been filled with K, as Ca
and Mg leached out over millennia, or have been abused by excessive
applications of Potassium chloride KCl fertilizers that “plug up” the exchange sites
on the edges of layered clays, or fill the spaces between the layers with K+.
Potassium just happens to be the perfect size to collapse the space between clay
layers so that Ca and Mg can no longer fit in between to attach to the (
- )
negative exchange sites. To restore a collapsed layer clay or one where the
exchange sites on the edges are plugged with K, the logical approach would be to
greatly increase the Calcium saturation in the soil with the aim of displacing K
from the layered-clay edges, and perhaps even expanding the layers that are
presently collapsed by displacing some of the interlayer K with Ca.
Gypsum, Calcium sulfate, is the best tool for opening collapsed layer clays or
displacing excess cations because it readily dissolves and dis-associates into
Ca
2+
and SO
4
2-
ions in the soil/water solution.Ahigh enough concentration of Ca
ions will tend to displace any other cation on the exchange sites; the displaced
ions will then either be taken up by plants and soil organisms or quickly associate
with free SO
4
2-
forming K
2
SO
4,
MgSO
4
, Na
2
SO
4
etc. which can readily be leached
to a lower soil horizon.