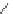Chemistry Reference
In-Depth Information
N
N
N
N
FVP
1050 °C
FVP
1050 °C
15
-
20%
Br
Br
94
95
96
Scheme 27 Synthesis of benzo[
g
]acecorannulene (91)[
122
]
Dibenzo[
a
,
g
]corannulene (88) was prepared from fluoranthene 9 under FVP
conditions at 1,000
C (Scheme
25
)[
117
]. Compound 88 was also accessed from
the same material by Pd-catalyzed intramolecular arylations [
16
]. In that report,
Scott and coworkers tested various palladium catalysts, bases, and reaction condi-
tions, and the best result (57% yield) was obtained under conditions employing the
combination of palladacycle/DBU/DMF at 150
C. A synthetic method has been
reported to prepare 7,10-disubstituted fluoranthenes, including 9, in good to excel-
lent yields by Rh-catalyzed [(2+2)+2]
cycloadditions of 1,8-di(ethynyl)
naphthalenes 87 with NBD [
118
].
Similar to dibenzo[
a
,
g
]corannulene (88), 1,2-dihydrocyclopenta[
b,c
]dibenzo-
[
g,m
]corannulene (90)[
119
] and
N
-octyldibenzo[
d
,
m
]-1,2-corannulimide (91)
[
120
] were synthesized by Pd-catalyzed cyclization (Scheme
26
). The OFET
devices of 91 show interesting electron and hole motilities, and may have potential
applications in organic electronic devices. Dibenz[
f,l
]acecorannulene (93)[
121
]
can be directly prepared from 92 under FVP conditions.
Benzo[
g
]acecorannulene (96) is an unexpected product generated from 95.
According to the successful synthesis of dibenzo[
a,g
]corannulene (88) from 7,10-
bis(2-bromophenyl)fluoranthene (9), an attempt was made to prepare
aza
-bowl 94
from 95. However,
this protocol gave 96 as the only identifiable product
(Scheme
27
)[
122
].
2.4.2 Structures and Properties
On the basis of crystallographic analysis, the corannulene cores in dibenzo[
a
,
g
]-
corannulene (88) and
N
-octyldibenzo[
d
,
m
]-1,2-corannulimide (91) are shallow
with a bowl depth of 0.83 and 0.65
, respectively [
120
,
123
]. The cyclopenta-
annulated buckybowl 90 is much deeper, and its bowl depth was determined as
1.03
Å
. The maximum POAV pyramidalization angle of 90 was found to be 10.7
,
which is very close to that of acecorannulene (69)[
119
]. However, the bowl
inversion barrier for 90 was experimentally estimated as 23.5-23.6 kcal/mol,
which is much smaller than that of acecorannulene (69, 27.6 kcal/mol).
Dibenzo[
a
,
g
]corannulene (88) and fullerenes C
60
and C
70
form cocrystals
[88·C
60
] and [(88)
2
·C
70
], respectively [
123
]. The bowl depth of the corannulene
moiety in dibenzocorannulene are slightly deceased upon co-crystallization, and the
Å